Diels–Alder cycloaddition reaction is one of the most powerful strategies for the construction of six-membered carbocyclic and heterocyclic systems, in most cases with high regio- and stereoselectivity. In this review, an insight into the most relevant advances on sustainable Diels–Alder reactions since 2010 is provided. Various environmentally benign solvent systems are discussed, namely bio-based derived solvents, polyethylene glycol, deep eutectic solvents, supercritical carbon dioxide, water and water-based aqueous systems.
- Diels–Alder reaction
- green solvent
- bio-based solvent
- deep eutectic solvents
- water
- green chemistry
1. Introduction
2. Bio-based solvents
Glycerol, the main by-product in the biodiesel industry, is a nontoxic, biodegradable, recyclable and inexpensive viscous liquid. These properties, allied with the high stability, biocompatibility and ability to dissolve organic compounds poorly miscible in water as well as inorganic compounds, make glycerol a valuable green solvent in synthetic organic chemistry [38,39,40] [38][39][40].
The three-component aza-Diels–Alder reaction of substituted anilines, aldehydes and electron-rich alkenes, also known as three-component imino-Diels–Alder reaction, or multicomponent Povarov reaction, is one of the most straightforward, efficient and atom-economical strategies towards complex cores starting from simple, inexpensive and available materials. Perin and coworkers explored the intramolecular version of this reaction for the catalyst-free synthesis of octahydroacridines starting from (R)-citronellal (1) and substituted arylamines 2 using glycerol as a recyclable and eco-friendly solvent (Scheme 1) [41] [41]. Cycloadducts 3 and 4 were obtained as diastereoisomeric mixtures in good to high yields (75–98%) and moderate cis-selectivity when the reaction was carried out at 90 °C. Cycloadducts 3/4 (R = H) were obtained in lower yield (62%) using water as solvent, whereas the reaction carried out in organic solvents afforded the corresponding adducts in only trace amounts. Due to the insolubility of 3 and 4 in glycerol, products could be removed from the reaction medium by decantation, and the solvent could be reused for further aza-Diels–Alder reactions without loss of activity.

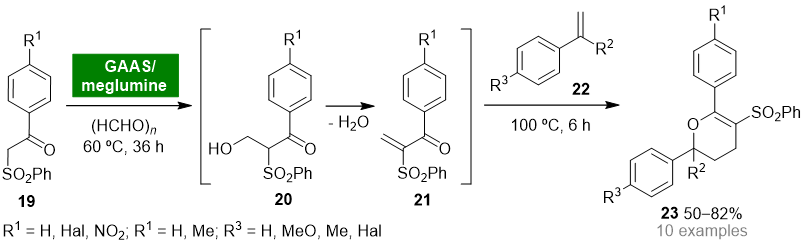
Gluconic acid (GA) can be obtained from biomass and possesses the ideal properties for being classified as a green and sustainable solvent (e.g., nontoxicity, biodegradability, recyclability, high boiling point, low vapor pressure) [42]. Due to the high solubility of gluconic acid in water, gluconic acid aqueous solutions (GAASs) have found wide application as solvent media for organic reactions, namely for the Knoevenagel condensation reaction. The Gu group reported the synthesis of 2H-pyrans by a one-pot multicomponent reaction between β-ketosulfones, formaldehyde and styrenes in a bio-based binary mixture solvent system composed of GAAS and a sugar-based organic base, meglumine [43]. The disclosed protocol involves the in situ generation of α-methylene-β-ketosulfones 21 through a Knoevenagel reaction of β-ketosulfones 19 and formaldehyde. Next, nucleophilic trap of 21 with styrenes 22 via oxa-Diels–Alder reaction afforded 2,6-diaryl-5-(phenylsulfonyl)-3,4-dihydro-2H-pyrans 23 in moderate to good yields (50–82%) (Scheme 2). The binary solvent system GAAS/meglumine proved to play a pivotal role in controlling the selectivity of the hydroxymethylation step. Moreover, the hydrophilic properties of bio-based solvent meglumine allowed it to be easily recycled and reused in the GAAS/meglumine system without significant loss of activity.
3. Polyethylene Glycol
Polyethylene glycol (PEG), HO–(CH2CH2O)n–H, is a biodegradable, nontoxic, odorless, neutral, nonvolatile and inexpensive water-soluble polymer that has found widespread application as a green reaction medium for several organic transformations [44][20]. The Kouznetsov group reported the diastereoselective synthesis of heterolignan-like 6,7-methylendioxy-tetrahydroquinolines via a BF3.OEt-catalyzed three-component Povarov reaction using clove bud essential oil as a renewable raw material and PEG-400 as green solvent (Scheme 3) [45][44]. Clove bud essential oil enriched with eugenol 24 (60.5%) was obtained by hydrodistillation of dried flower buds and then subjected to a solid base-catalyzed isomerization to give trans/cis-isoeugenol 25, which could be used as a dienophile in the multicomponent hetero-Diels–Alder reaction without further purification. The reaction of 25 with aldimines generated in situ from substituted benzaldehydes 27 and 3,4-(methylendioxy)aniline (26) afforded trans-2,4-diaryl-1,2,3,4-tetrahydroquinolines 28 as racemic mixtures in moderate yields (35–55%). The reaction with phthalaldehydic acid (29) afforded isoindolo[2,1-a]quinolin-11(5H)-one 30 via an intramolecular condensation of the initially generated NH-tetrahydroquinoline core with the o-carboxylic acid function leading to the formation of the γ-lactam ring. It is noteworthy that these reactions also worked using acetonitrile as solvent media; however, less solvent volume and reduced reaction times were required when using PEG-400.
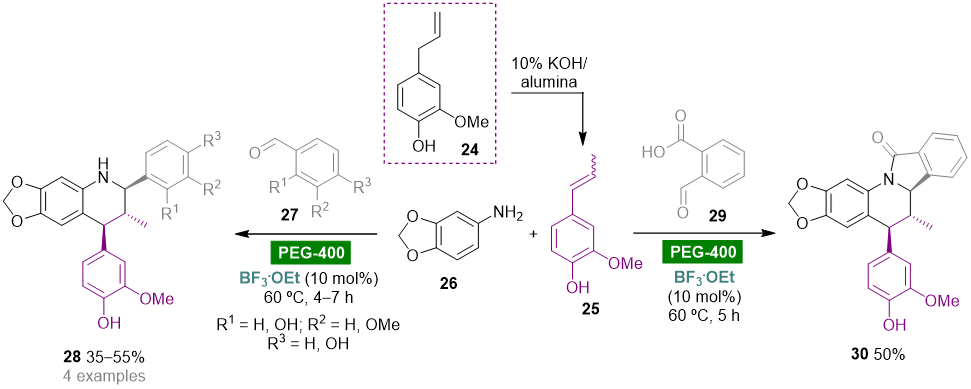
Scheme 3.
BF
3.
OEt-catalyzed one-pot multicomponent aza-Diels–Alder reaction of 3,4-(methylendioxy)aniline, aromatic aldehydes and
trans
/
cis
-isoeugenol in PEG-400.
4. Organic Carbonates
Propylene carbonate (PC) is a polar aprotic solvent that can be obtained from propylene oxide and carbon dioxide, a renewable source of carbon, in a 100% atom economy reaction with relevance regarding the development of CO2 fixation processes. The noncorrosive, nontoxic, odorless and biodegradable properties of PC, allied with high boiling point, low vapor pressure and low cost, make this solvent a green and sustainable alternative to conventional organic solvents [46][45]. The Povarov reaction has also been explored using PC as an environmentally friendly solvent [47][46]. The one-pot iodine-catalyzed reaction of mono- or disubstituted anilines 2, aromatic aldehydes 5 and isoeugenol (45) carried out at room temperature using PC as solvent medium afforded functionalized tetrahydroquinolines 46 in good to high yields (77–95%) and high diastereoselectivity (dr up to >99:1) (Scheme 4). The same cycloadducts were obtained using organic solvents, albeit in low yields and requiring longer reaction times. It is noteworthy that, in general, products precipitated from the reaction medium and were purified by recrystallization.
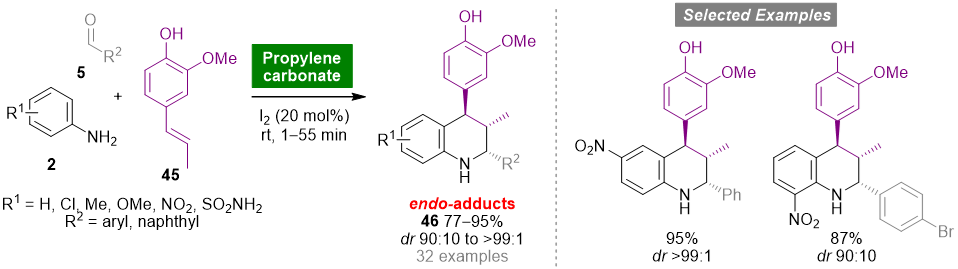
Scheme 4. Iodine-catalyzed one-pot multicomponent aza-Diels–Alder reaction of anilines, aromatic aldehydes and isoeugenol in propylene carbonate.
5. Deep Eutectic Solvents
First introduced by Abbot [48][47], deep eutectic solvents (DESs) are low melting mixtures obtained by combination of at least two components, a hydrogen bond acceptor (HBA), generally a quaternary ammonium or metal salt, and a hydrogen bond donor (HBD), to form a eutectic phase via hydrogen bond interactions. DESs are characterized by a melting point lower than those of the single components. The properties of DESs are very similar to those of room-temperature ionic liquids; however, the main difference from ionic liquids is that DESs also contain an organic molecular component, the HBD (e.g., urea, amide, polyol), generally as a major component. Due to their low vapor pressure, nonflammability, thermal and chemical stability, nontoxicity, biodegradability, recyclability and low price, DESs have emerged as green and sustainable media in different areas of chemical research [49,50][48][49][50][51], namely organic synthesis and catalysis [51,52] [23][24][25][26].
Garcia-Álvarez’s group reported a one-pot tandem cycloisomerization/Diels–Alder reaction using a ChCl (choline chloride)-based eutectic mixture as solvent [53][52]. The protocol involves the in situ generation of furans 59 by cycloisomerization of (Z)-enynols 57 using a ChCl/Gly (1:2) eutectic mixture as solvent and bis(iminophosphorane)-Au(i) complex 58 as catalyst (Scheme 5). The Diels–Alder reaction of furans 59 with activated alkynes 60 afforded 7-oxanorbornadienes 61, whereas the reaction with activated alkenes, 55b or 62, afforded selectively exo-7-oxanorbornenes 63. The authors have demonstrated that complex 58 is crucial for the cycloisomerization step; however, it does not participate in the cycloaddition step.
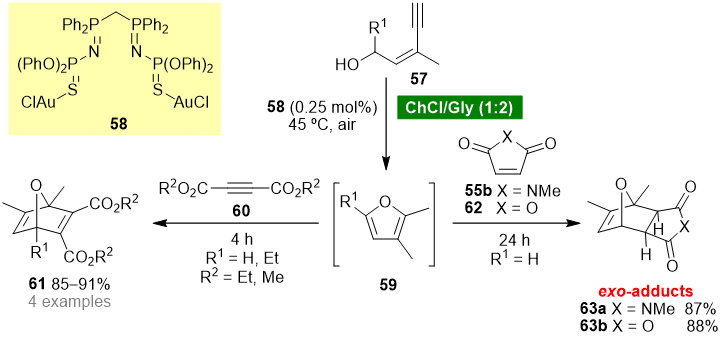
Scheme 5. One-pot tandem cycloisomerization/Diels–Alder reaction of (Z)-enynols in a ChCl/Gly deep eutectic solvent.
6. Supercritical Carbon Dioxide
Supercritical carbon dioxide (scCO2) is an abundant, low-cost, nontoxic and nonflammable fluid. The physical properties of scCO2 are intermediate between the gas and the liquid phases. These properties can be tuned by changing pressure and temperature; in particular, changes close to the critical point enable drastic changes in density, viscosity and diffusion. Among supercritical fluids, scCO2 has received special attention since it is readily accessible at a low critical temperature (Tc = 31 °C) and moderate critical pressure (Pc = 75.8 bar) [54]. In addition, scCO2 has the ability to dissolve organic compounds and can be easily removed from the reaction mixture, making it a sustainable alternative to conventional organic solvents in synthetic transformations [55,56,57,58]. Keshtov et al. disclosed a green approach for the synthesis of photoluminescent polymers based on phenyl-substituted polyfluorenes using scCO2 as the solvent medium [59]. Phenylated polyfluorenes 88 were synthesized through a catalyst-free Diels–Alder reaction of fluorene-containing bis(tetraarylcyclopentadienone) monomer 86, acting as diene, with bis(acetylenes) 87 acting as 2π-component (Scheme 6). Phenyl-substituted polyfluorenes synthesized using scCO2 as solvent showed similar properties to those synthesized using chloronaphthalene as solvent, demonstrating that scCO2 is a suitable alternative to organic solvents for the synthesis of phenylated polyfluorenes via the Diels–Alder reaction.
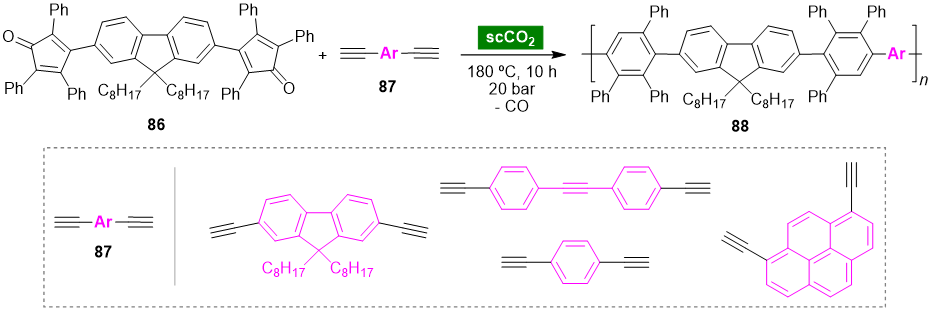
Scheme 6. Diels–Alder reaction of fluorene-containing bis(tetraarylcyclopentadienone) and bis(acetylenes) in scCO2.
7. Water
The use of water as an alternative benign and sustainable solvent in promoting Diels–Alder cycloaddition reactions has been widely explored since the seminal work of Breslow’s and Grieco’s groups [60,61,62,63]. The remarkable rate enhancements and selectivities achieved on moving from conventional organic solvents to aqueous conditions are the major driving force behind the growing interest in the study of the Diels–Alder reaction in this medium [64,65].
7.1. The Water Effect: Experimental and Theoretical Studies
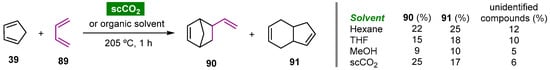
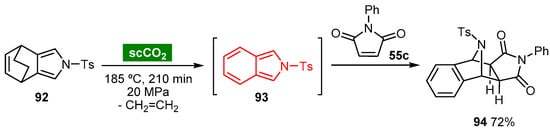
7.2. Noncatalyzed Diels–Alder Cycloaddition Reactions
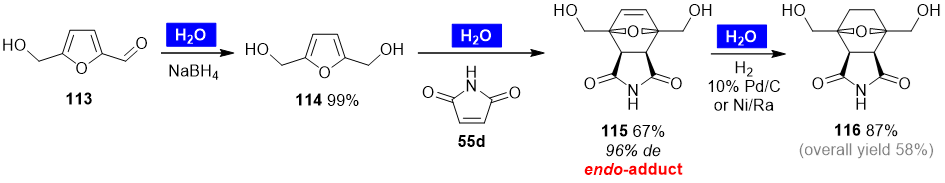
Ananikov and coworkers disclosed a three-step synthesis of polycyclic compound 116 from bio-based 5-(hydroxymethyl)furfural (HMF) (113) involving a Diels–Alder cycloaddition step (Scheme 8) [68]. Computational calculations demonstrated that the Diels–Alder reaction of 2,5-bis(hydroxymethyl)furan (BHMF) (114) and maleimide is energetically more favorable than that of HMF (113) with the same dienophile. Thus, HMF (113) was reduced to BHMF (114) in aqueous solution. Subsequent cycloaddition reaction of 114 with maleimide (55d) in water furnished compound 115 diastereoselectively (96% de) as the endo-adduct in 67% yield. To prevent the retro-Diels–Alder transformation, 115 was further reduced, giving compound 116 in 87% yield (58% overall yield). It is noteworthy that the three-step one-pot protocol in water, starting from HMF (113), proved to be more efficient, allowing the synthesis of 116 in 73% yield.
7.3. Catalyzed Diels–Alder Cycloaddition Reactions
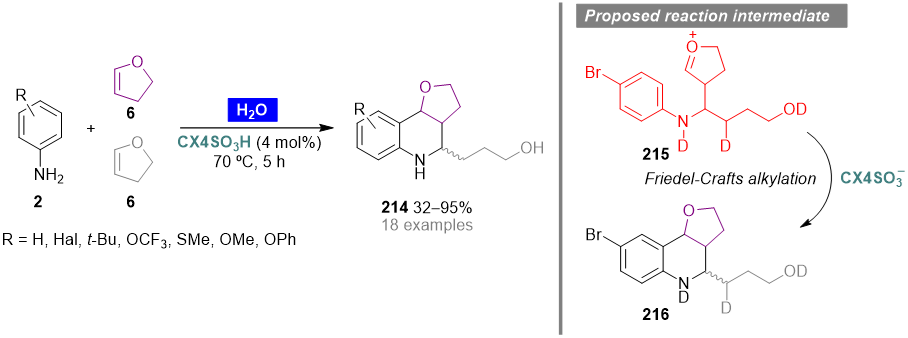
The synthesis of furano[3,2-c]-1,2,3,4-tetrahydroquinolines involving the multicomponent Povarov reaction between anilines 2 and two equivalents of 2,3-dihydrofuran (6), has been recently reported by Fernandes and coworkers (Scheme 9) [69]. Using water as solvent and p-sulfonic acid calix[4]arene (CX4SO3H) as organocatalyst, a series of tetrahydroquinoline derivatives 214 were prepared in moderate to excellent yield, with aniline and 4-halo-anilines providing the target products in the highest yields (85–95%). The catalyst CX4SO3H could be reused up to four times while maintaining the catalytic activity. Isotopic labeling experiments involving the synthesis of furano[3,2-c]-1,2,3,4-tetrahydroquinoline 216, supported by NMR and mass spectroscopy data, were crucial for the validation of the reaction mechanism. The reaction seems to evolve through a stepwise sequence via ionic intermediates originating oxonium ion 215, which undergoes an intramolecular electrophilic aromatic substitution, under CX4SO3H catalysis, to afford the final products.
7.4. Asymmetric Diels–Alder Cycloaddition Reactions
The “on-water” organocatalyzed [4+2] cycloaddition reaction of acyclic enones with nitro dienes and allylidene malononitriles gave access to a plethora of functionalized chiral cyclohexanones, using cinchona alkaloid-based primary amines (e.g., 249 and 252) and benzoic acid as the organocatalytic system (Scheme 10) [70,71]. Unsaturated methyl ketones 247 reacted smoothly with primary amine catalyst 249 in the presence of benzoic acid to give the corresponding enamine, which undergoes an endo [4+2] cycloaddition with nitro dienes 248 to yield the corresponding 3,4,5-trisubstituted cyclohexanones 250 in good yield, with good diastereoselectivities and excellent enantioselectivities. On the other hand, the use of allylidene malononitriles 251 as dienophiles led to the enantioselective synthesis of cyclohexanones 253 with two stereocenters and an all-carbon quaternary center. Allylidene cyanoacetates were also suitable dienophiles for these transformations.
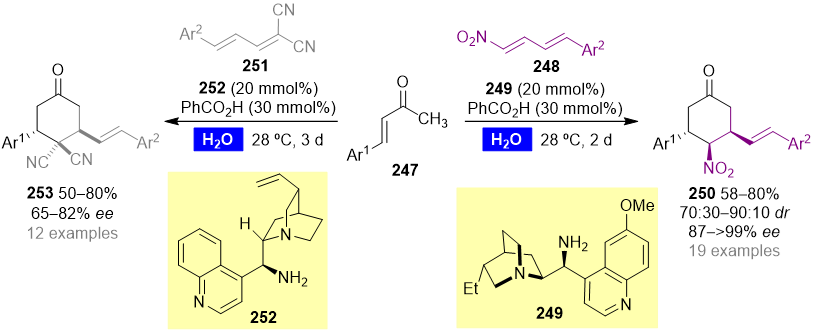
Scheme 10. Organocatalyzed enantioselective synthesis of functionalized cyclohexanones in water.
8. Conclusions
The Diels–Alder reaction remains one of the most commonly employed reactions for the rapid construction of carbocyclic and heterocyclic compounds, including the synthesis of natural products. In this review, the most significant advances on the Diels–Alder reaction in environmentally benign solvent systems were highlighted.
The fast-growing green chemistry research area has driven successful developments on this topic, upgrading this traditional reaction to the present sustainable needs. Notably, high levels of chemical efficiency and selectivity were achieved in most of the described methodologies, with the use of these sustainable solvent media leading to shorter reaction times and simpler workup procedures than the use of conventional organic solvents. Additionally, the Lewis acid catalysis and organocatalysis combined with nonconventional heating methods have broadened the scope of these methodologies. Despite the recent progress, further developments on sustainable enantioselective synthetic approaches are required. The development of highly active catalysts, namely functionalized heterogeneous catalysts with well-defined structures, suitable to a wider range of substrates and solvents, is needed to overcome some of the current challenges. Therefore, innovative strategies for the design of new catalytic systems with enhanced properties can be expected in the near future.
References
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227.
- Díaz-Álvarez, A.E.; Francos, J.; Crochet, P.; Cadierno, V. Recent advances in the use of glycerol as green solvent for synthetic organic chemistry. Curr. Green Chem. 2014, 1, 51–65.
- Abd-Elmonem, M.; Mekheimer, R.A.; Hayallah, A.M.; Abo-Elsoud, F.; Sadek, K.U. Recent advances in the utility of glycerol as a benign and biodegradable medium in heterocyclic synthesis. Curr. Org. Chem. 2019, 23, 3226–3246.
- Nascimento, J.E.R.; Barcellos, A.M.; Sachini, M.; Perin, G.; Lenardão, E.J.; Alves, D.; Jacob, R.G.; Missau, F. Catalyst-free synthesis of octahydroacridines using glycerol as recyclable solvent. Tetrahedron Lett. 2011, 52, 2571–2574.
- Lim, H.Y.; Dolzhenko, A.V. Gluconic acid aqueous solution: A bio-based catalytic medium for organic synthesis. Sustain. Chem. Pharm. 2021, 21, 100443.
- Yang, J.; Li, H.; Li, M.; Peng, J.; Gu, Y. Multicomponent reactions of β-ketosulfones and formaldeyde in a bio-based binary mixture solvent system composed of meglumine and gluconic acid aqueous solution. Adv. Synth. Catal. 2012, 354, 688–700.
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82.
- Arenas, D.R.M.; Ruíz, F.A.R.; Kouznetsov, V.V. Highly diastereoselective synthesis of new heterolignan-like 6,7-methylendioxy-tetrahydroquinolines using the clove bud essential oil as raw material. Tetrahedron Lett. 2011, 52, 1388–1391.
- Forero, J.S.B.; Muñoz, J.A.H.; Jones, J.; da Silva, F.M. Propylene carbonate in organic synthesis: Exploring its potential as a green solvent. Curr. Org. Synth. 2016, 13, 834–846.
- Forero, J.S.B.; de Carvalho, E.M.; Junior, J.J.; da Silva, F.M. Facile, efficient diastereoselective synthesis of tetrahydroquinoline scaffolds using propylene carbonate as an eco-friendly solvent. Curr. Org. Synth. 2015, 12, 102–107.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Zhang, Q.; Vigier, K.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082.
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, properties, and applications. Chem. Soc. Rev. 2021, 50, 8596–8638.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285.
- Wang, A.; Zheng, X.; Zhao, Z.; Li, C.; Zheng, X. Deep eutectic solvents to organic synthesis. Prog. Chem. 2014, 26, 784–795.
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704.
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386.
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632.
- Vidal, C.; Merz, L.; García-Álvarez, J. Deep eutectic solvents: Biorenewable reaction media for Au(I)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels–Alder reactions. Green Chem. 2015, 17, 3870–3878.
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical fluids as solvents for chemical and materials processing. Nature 1996, 383, 313–318.
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 1878–1895.
- Kaupp, G. Reactions in supercritical carbon dioxide. Angew. Chem. Int. Ed. 1994, 33, 1452–1455.
- Oakes, R.S.; Clifford, A.A.; Rayner, C.M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc. Perkin Trans. 2001, 1, 917–941.
- Licence, P.; Ke, J.; Sokolova, M.; Ross, S.K.; Poliakoff, M. Chemical reactions in supercritical carbon dioxide: From laboratory to commercial plant. Green Chem. 2003, 5, 99–104.
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191.
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778.
- Keshtov, M.L.; Mal’tsev, E.I.; Lopatin, A.M.; Nikitin, L.N.; Marochkin, D.V.; Perevalov, V.P.; Petrovskii, P.V.; Khokhlov, A.R. Photoluminescent phenylated polyfluorenes synthesized in an organic solvent and supercritical carbon dioxide. Polym. Sci. Ser. B 2012, 54, 106–114.
- Meng, F.-Q.; Feng, X.-J.; Wang, W.-H.; Bao, M. Synthesis of 5-vinyl-2-norbornene through Diels–Alder reaction of cyclopentadiene with 1,3-butadiene in supercritical carbon dioxide. Chin. Chem. Lett. 2017, 28, 900–904.
- Ito, S.; Akaki, M.; Shinozaki, Y.; Iwabe, Y.; Furuya, M.; Tobata, M.; Roppongi, M.; Sato, T.; Itoh, N.; Oba, T. Efficient synthesis of isoindoles using supercritical carbon dioxide. Tetrahedron Lett. 2017, 58, 1338–1342.
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778.
- Hailes, H.C. Reaction solvent selection: The potential of water as a solvent for organic transformations. Org. Process Res. Dev. 2007, 11, 114–120.
- Chanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748.
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551.
- Butler, R.N.; Coyne, A.G. Organic synthesis reactions on-water at the organic-liquid water interface. Org. Biomol. Chem. 2016, 14, 9945–9960.
- Kitanosono, T.; Masuda, K.; Xu, P.Y.; Kobayashi, S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2018, 118, 679–746.
- Bhowmick, K.C.; Bihani, M.; Zhao, J.C.-G. Organocatalyzed asymmetric Diels–Alder reactions in aqueous or semi-aqueous media. Mini Rev. Org. Chem. 2018, 15, 3–19.
