Obesity disease results from a dysfunctional modulation of the energy balance whose master regulator is the central nervous system. Consistently, the prevalence of obesity is higher among individuals who experienced early life stress (ELS). Oxytocin, a hypothalamic neurohormone, regulates the energy balance and modulates social, emotional, and eating behaviors, exerting both central and peripheral actions. Oxytocin closely cooperates with leptin in regulating energy homeostasis. Based on the available data, alterations in the oxytocin system may in part mediate the ELS-induced susceptibility to obesity.
- obesity
- early life stress (ELS)
- oxytocin
1.Obesity: epidemiology, etiopathophysiology and early development
Obesity, defined by a body mass index (BMI) ≥30,0 kg/m
2, is a multifactorial, chronic, and relapsing disease that has spread as a real pandemic during the last decades (Bluher 2019; Ward et al. 2019). Obesity prevalence has nearly tripled since 1975 and its incidence is expected to further increase during these coming years (EASO 2020; Bluher 2019; Ward et al. 2019). This estimate does not surprise as the incidence of obesity among children is steeply rising (WHO 2020), and such condition is usually maintained throughout adulthood (Geserick et al. 2018). Obesity is associated with a higher risk of developing over 200 medical complications, among which insulin resistance, type 2 diabetes mellitus, hypertension, metabolic syndrome, cardiovascular diseases, and several types of cancer. As a consequence, obesity is recognized as the fifth leading cause of death worldwide, and as a burden for the global health-care systems (WHO 2018; Bray et al. 2017; Colleluori et al. 2021).
From a biological standpoint, obesity disease results from the inability to guarantee body energy homeostasis, an impairment referred to as energy balance dysfunction. Such concept is often simplified and considered as the result of excessive energy intake (eating) and low energy expenditure (physical activity), hence of an “unhealthy lifestyle”. However, the etiology of obesity is complex and involves the interaction of numerous elements that makes the disease multifactorial (Science 2007). Based on the Foresight Study, environmental (i.e., food industry, pollution, education, culture, access to healthcare), psychological (individual and social) and biological (genetic, epigenetic, endocrinological) factors not only play a role in determining obesity risk, but also positively and negatively influence each other in triggering the disease and its morbidity (Science 2007). Importantly, all those causal factors lead to the dysfunction in the ability to regulate the energy balance (energy intake and expenditure).
The modulation of the energy balance occurs in the central nervous system (CNS) (Giordano 2018; Leng et al[10][11][12][13]. 2017; Andermann and Lowell 2017; Myers et al. 2021). The master regulator is the hypothalamus where all signals from other brain areas and periphery are integrated and translated into specific behavioral, autonomic, and endocrine outputs (Giordano 2018; Leng et al[10][11][12][14][15]. 2017; Leng 2018; Waterson and Horvath 2015; Andermann and Lowell 2017). The crucial role of the CNS in obesity susceptibility is well documented by recent genome wide association studies that implicate pathways related to synaptic function, extracellular matrix composition, and glutamate signaling (Locke et al. 2015) [16], as well as brain G protein-coupled receptors, as primary in determining BMI variations (Akbari et al [17]. 2021).
The maturation of the central neural circuitries involved in energy balance control is not completed at birth but occurs during early life. In mammals, postnatal ages are denoted by critical developmental periods during which organs and neural systems are highly plastic. In this timeframe, adverse nutritional, social, and environmental cues may program body metabolism to maximize energy accrual to face hostile conditions. Consistently, a higher prevalence of obesity among individuals exposed to early life stress (ELS) during both the pre- and postnatal periods is documented (Moreno-Mendez 2020) [18].
In rodents, while thermogenic brown adipose tissue (BAT) is already present at birth to sustain pups’ survival, white adipose tissue (WAT) progressively develops during the first postnatal weeks. This developmental timing may represent an evolutionary strategy needed to shape and adjust metabolic functions to environmental cues, e.g., maternal care and food availability. Obesity disease is hence rarely due to environmental and/or genetic factors alone and results from the interaction between the individual’s biological characteristics and the environment in which he lives in (Bluher 2019). For this reason obesity is recognized as a preventable disease (Bluher 2019).
The study of the cellular and molecular mechanisms at the basis of the postnatal differentiation of the energy homeostasis circuitry may shed light on the etiopathological basis of many forms of obesity and may offer new targets for intervention and prevention. Given the strong association between disorganized/inconstant maternal care and obesity risk (Miller, Chen, and Parker 2011; Miller and Lumeng 2018), the identification of the biological pathways and mediators of such link is of relevance. In this context, the Oxt system is a critical candidate given its role in maternal bond, response to stress and feeding behavior (Carter 2017; Lawson et al. 2020; Baracz, Everett, and Cornish 2020). In this review article, we report evidence from the literature documenting the effect of ELS (specifically postnatal stress induced by disorganized/inconstant maternal care) on obesity vulnerability, with a particular focus on Oxytocin (Oxt) and Leptin roles in rodent models. We emphasize the existing gaps in the literature and highlight promising research direction worthy of exploration.
2. Early life stress
Clinical observations and researches of distinguished psychiatrists and pediatricians such as John Bowlby and Donald Winnicott, emphasized the crucial role of early life experiences, in particular the relationship between infants and mothers (or caregiver), as determinants of the psychological health in adulthood (Bowlby 1969; Winnicott 1957). Specifically, a disorganized care attitude towards the offspring results in an abnormal attachment/bonding relation with the parental figure, in turn associated with the development of dysfunctional behaviors (e.g., depression, drug addiction and eating disorders) in adulthood (Bowlby 1969; Miller and Lumeng 2018; Miller, Chen, and Parker 2011; Baracz, Everett, and Cornish 2020). During early life, parental-offspring interaction is a crucial element impacting offspring developmental trajectories (Nelson and Gabard-Durnam 2020). In animal models, ELS is usually reproduced by maternal separation (MS), a strategy consisting of the separation of pups from their dam for 180 minutes per day, or by limiting the nesting and bedding material (LN), a procedure resulting in disorganized maternal care, hence stress among pups (Rice et al. 2008; Molet et al. 2014). Maternal care anomalies lead to abnormal developmental courses underlined by complex and only partially described neuroendocrine cascades (Curley and Champagne 2016). Growth in an unpredictable environment, where maternal care is not constantly guaranteed, may result in a neurometabolic programming aimed at maximizing energy accrual and minimizing its waste, a possible adapting strategy to face hostile conditions. As a result, also the rewarding value of feeding may be enhanced (Leng 2018).
Although acute and chronic stress effects on eating behavior and metabolic health have been extensively studied in adulthood, the mechanism by which ELS affects adult eating behavior and metabolic health is not clear (Miller, Chen, and Parker 2011). Exposure to stress during early life development is associated with overweight and obesity in adult humans (Miller, Chen, and Parker 2011; Miller and Lumeng 2018). ELS is known to negatively impact the neurobiological, cognitive, social-emotional, behavioral, and physical development in humans and animal models. In the timeframe characterizing early development, known as critical period, infants’ brain is extremely plastic; phenomena of pruning and synaptogenesis are highly prevalent and necessary to shape neuronal circuit and to complete brain maturation, which concludes only after adolescence (Nelson and Gabard-Durnam 2020). In this developmental process, a crucial role is also played by glial cells which actively modulate synaptic formation, pruning, neurovascular coupling, and phagocytosis, and act as metabolic sensors (Garcia-Caceres et al. 2019), integrating a multitude of signals to adapt to the environment (Abbink et al. 2019). This period of high plasticity is deeply influenced by life experiences and is characterized by an elevated vulnerability to negative stimuli which can shape the way the neuronal network is built (Nelson and Gabard-Durnam 2020; Curley and Champagne 2016). However, relatively little is known about how these critical periods can impact the development of peripheral organ (e.g., adipose tissue) and the crosstalk between these organs with the CNS.
Although ELS biological effects on cognitive function, anxiety and depression-like behaviors have been widely investigated (Ruiz et al. 2018; Goodwill et al. 2019; Novick et al. 2018), their consequences on the central regulation of the energy balance, eating behavior, adipose tissue development, and metabolic health have not been explored in depth and only a handful of studies, with very heterogeneous experimental design, are available on the topic (Miller, Chen, and Parker 2011; Ruiz et al. 2018; Paternain et al. 2012; Maniam et al. 2015; Yam et al. 2017; Ryu et al. 2009; Eller et al. 2020; de Lima et al. 2020).
3.Oxytocin: the neuroendocrine hub of social bonding, stress, eating behavior, and metabolic health
In the study of early adversity determined by abnormal infant care, particularly pertinent is the research on the neurohormone Oxt. Produced by neurons located in the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei (Figure 1 A-E), Oxt plays a pivotal role in the regulation of a variety of behaviors including social, emotional, sexual, eating, and addiction behaviors (Leng 2018) [14]. Oxt is produced by both magnocellular and parvocellular neurons: the formers are contained in PVN and SON and mainly project to the neurohypophysis where Oxt is released into the circulation, while the latters, mainly contained in the PVN but also scattered in other hypothalamic and extrahypothalamic areas, project to different hindbrain regions e.g., solitary tract nucleus (Williamson et al[32][33]. ; Jurek and Neumann 2018). Interestingly, specialized PVN and SON magnocellular Oxt neurons develop axon collaterals projecting to forebrain limbic regions (e.g., prefrontal cortex, nucleus accumbens, anterior and central amygdala, bed nucleus of stria terminalis -BNST-, hippocampus) (Figure 2 A-F), a finding revealed only in advanced vertebrates and thought to have developed together with the social and emotional behavioral complexity of species.
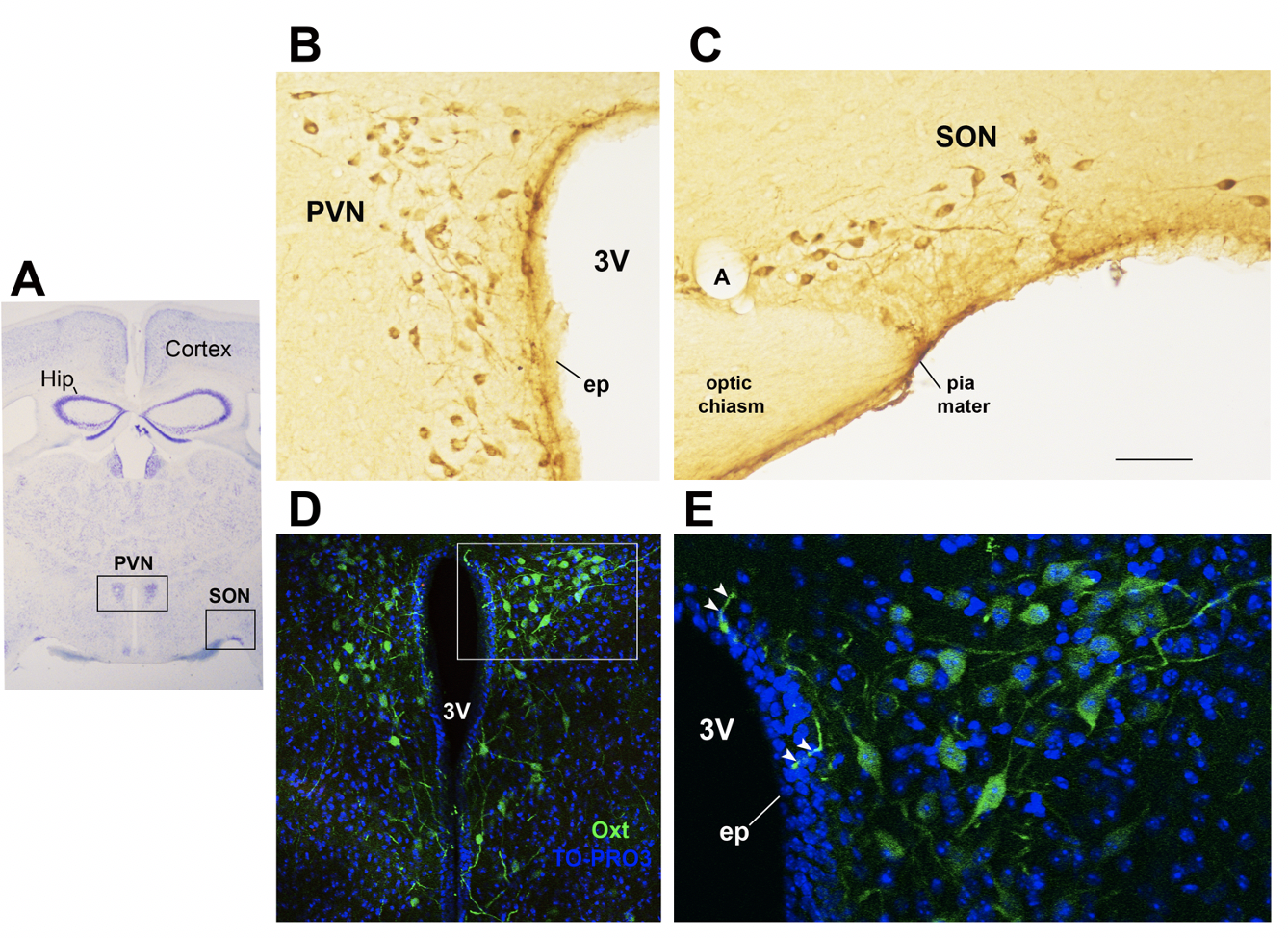
Figure 1. Oxytocinergic neurons in the paraventricular (PVN) and supraoptic (SON) hypothalamic nuclei
(A) Light microscopy (LM): Nissl-stained brain coronal section of Bregma − 0.94 mm; PVN: hypothalamic paraventricular nucleus; SON: hypothalamic supraoptic nucleus; Hip: Hippocampus. (B) LM: peroxidase immunohistochemistry of Oxytocin (Oxt) positive neurons in proximity of the ependymal layer (ep) of the third ventricle (3V) in the PVN. (C) Peroxidase immunohistochemistry of Oxt neurons in SON; A: artery. (D) Double-label confocal microscopy of Oxt neurons (green) and cellular nuclei (blue TO-PRO3 staining) in the PVN. Panel (E) is an enlargement of the area framed in (D), showing Oxt positive neurons and their projections (arrowheads) reaching and contacting the ependymal cells and the cerebrospinal fluid of the third ventricle (3V). All figures refer to a 6 months old male C57BL/6 mouse. Bregma reference sections refer to “The mouse brain atlas” by Paxinos and Franklin. The scale bar was included in C only, and corresponds to different mm in each figure as follows: in A: 1500mm; in B and C: 45mm; in D: 120 mm; in E: 40mm. All figures are original and are not published elsewhere; for methodological details, please refer to supplementary material 1.
Oxt stimulates maternal care, maternal-infant attachment, and social bonding and is capable of attenuating stress response, displaying anxiolytic and antidepressant properties (Leng 2018; Carter 2017)[14][21]. Recent works on the neurobiological basis of attachment, coupled with studies on children adopted from orphanages, suggest that there may be a sensitive period for the development of oxytocin-dopamine connections (particularly in the nucleus accumbes of the striatum) which exert enduring effects on the neurobiology of social relationships (Feldman 2017)[34], including their ability to physiologically buffer stress (Kim et al. 2017) [35].
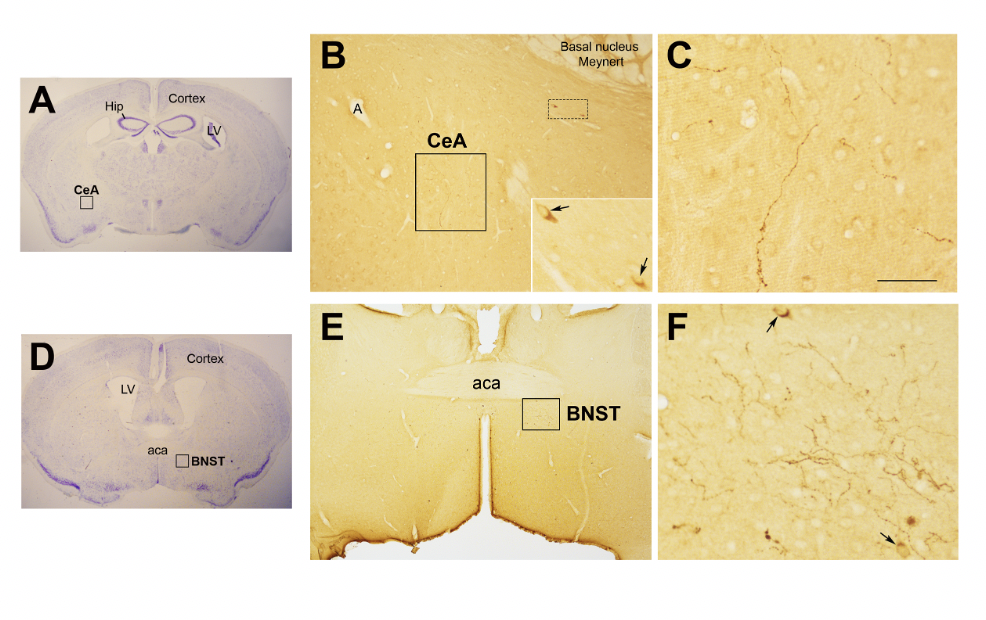
Figure 2. Oxytocinergic projections in the central nuclei of the amygdala (CeA) and in the bed nucleus of the stria terminalis (BNST).
(A) Light microscopy (LM): Nissl-stained brain coronal section of Bregma − 0.94 mm; Hip: hippocampus; LV: lateral ventricle; CeA: central nuclei of amygdala. (B) LM: peroxidase immunohistochemistry of Oxytocin (Oxt), showing oxytocinergic fibers (squared area) and the presence of two parvocellular Oxt neurons (dotted squared area) in CeA. Inset is the enlargement of the dotted squared area where Oxt positive neurons are indicated by arrows. (C) Enlargement of the squared area in (B) rich in Oxt positive fibers. (D) LM: Nissl-stained brain coronal section of Bregma 0.02 mm; BNST: bed nucleus of stria terminalis; aca, anterior commissure. (E) LM: peroxidase immunohistochemistry of oxytocinergic projections in the BNST. (F) Enlargement of the squared area in (E) showing the presence of Oxt neurons (arrows) and Oxt projections in BNST. All figures refer to a 6 months old male C57BL/6 mouse. Bregma reference sections refer to “The mouse brain atlas” by Paxinos and Franklin. The scale bar was included in C only, and corresponds to different mm in each figure as follows: in A and D: 2300mm; B: 200mm, inset 50mm; in C: 50mm; in E: 300mm; in F: 50mm. All figures are original and are not published elsewhere; for methodological details, please refer to supplementary material 1.
Interestingly, Oxt also ratteducnuates addictive behaviors and inhibits appetite (Feldman 2017)[34]. Consistent witly, an increasing numberh these data, there is a mounting body of evidence emphasizepointing at Oxt role in the promotion of ng weight loss and metabolic aamelioration in condition of ong obesity (Ding, Leow, and Magkos 2019; Lawson et al. 2020; Lawson-related metabolic dysfunctions 2017; Zhang et al[22][36][37][38]. 2013). Intranasal Oxt administration is currently being tested for the treatment of obesity as this route facilitates thean increase in the central concentrations of the nonapeptide through channels surrounding the trigeminal and olfactory nerve fibers (McCormack 2022; Lawson 2022; Espinoza 2019)[39][40][41]. Considering the complex and multiple Oxt functions of Oxt, which span from maternal-offspringaffects aspects as diverse as mother–infant bonding, eating behavior, and stress response, theits potential role played by this neurohormone in in determining the impact of ELS on eating behavior and metabolic health deserves further investigation.
4.Oxytocin system: early development and impact of ELS
Studies conducted on rodents demonstrated that early adverse experiences could have a profound impact on the way the oxytocin system is shaped. Oxt neurons progressively increase from PND 2 to 21, reaching maturation by the second postnatal week (Yamamoto et al[42]. 2004). On the other side, oxytocin neuron axons reach their targets only at early adulthood, when the Oxt receptor (Oxtr) is already widely expressed in different regions (detectable from embryonic day 14 in females and at PND 2 in males) (Jurek and Neumann 2018)[33]. Importantly, Oxt synthesizing neurons play a fundamental role in their own maturation: local Oxt release activates Oxtr expressed on Oxt neurons resulting in further Oxt discharge, a phenomenon that evidences how variation in Oxt levels during this period can deeply impact these neurons maturation (Yamamoto et al[14][43][42]. 2004; Leng, Caquineau, and Sabatier 2005; Leng 2018). It is possible that postnatal environmental stimuli affects Oxt production, hence the development of the Oxt system, with the final objective to retain only functional pathways necessary to face external conditions. This phenomenon may also explain the interindividual variability in Oxt neurons projection revealed by studies mapping the Oxt system (Liao et al[44]. 2020). In addition, different spatio-temporal Oxtr expression patterns during development have been described in males and females, suggesting a distinct and sex-specific sensitivity to Oxt levels during critical periods (Jurek and Neumann 2018) [33].
Alteration in Oxtr expression and binding have been detected in several brain regions in response to variation in maternal care in different animal models (Curley and Champagne 2016; Francis, Champagne, and Meaney 2000)[29][45]. Maternal high licking and grooming (LG) result in increased Oxt expression at PND13, while low LG leads to reduced Oxtr protein levels and receptor binding in several female’s brain regions (e.g., PVN, central nucleus of the amygdala) (Francis, Champagne, and Meaney 2000; Francis et al [29][46][45]. 2002; Curley and Champagne 2016). Although, Tsuda et al., documented a higher number of Oxt positive cells in the PVN of adult male mice exposed to MS (Tsuda [47], Yamaguchi, and Ogawa 2011), other studies reported a lower number of these cells in SON and PVN following a similar protocol (Kim et al[48][35][49]. 2017; Veenema, Bredewold, and Neumann 2007; Wei et al. 2021). Oxt changes in response to early adversity are generally in the direction of blunted circulating Oxt levels (Kim et al. 2017)[35]. However, Oxt levels may rice in response to a prolonged exposure to adverse stimuli to protect the system from the deleterious effects of stress (Kim et al[35]. 2017). .
A meta-analyses performed by Ellis and colleagues documented lower plasma OXT levels and reduced or negative response to OXT intranasal administration among individuals who expereinced childhood adversity (Ellis et al[50]. 2021). In addition, an unsecure attachment style was associated with lower OXTR expression in the peripheral blood mononuclear cells of women (Krause et al. 2018) [51]. Based on this evidence, early experience shapes the adult Oxt system and manipulation of maternal care from infancy confers enduring changes in the Oxtr expression profile, a phenomenon whose mechanisms and implications are not fully elucidated.
5.ELS, Oxytocin, eating behavior and metabolic health: future research directions
Recent studies documented the association between the alteration in the Oxt system induced by ELS and the development of cardiovascular diseases (Wigger et al[52][53][54]. 2020; McCook et al. 2021; Szczepanska-Sadowska et al. 2021). However, an important gap present in the literature concerns the investigation oflittle is known about the link between Oxt, ELS, and metabolic health. While tdata on the relationship between Oxt and ELSELS and Oxt and between ELS and metabolic health have been scarcely investigatedare scanty, evidence linking ELS to metabolic health, focusing on the Oxt system, hais not been documented in the literature. Specifically, it is unclear how ELS induces changes in the Oxt action of and sensitivity to Oxt and how these changes influence adipose tissue development, adulthood energy balance, metabolic health, and feeding behavior is not clear. Furthermore, ss ince greater attention has been paid on the study of the effect of Oxt manipulation during early development (Yamamoto et al. 2004), the function of endogenous Oxt role during such period remains poorly examined (Baracz, Everett, and Cornish 2020). Diadulthood. The sex-dependent different evidence emphasize how spatio-temporal Oxtr expression varies in response to environmental cues, especially during critical periods. Several authors believe that Oxtr may be a developmental plasticity gene that serves as a transducer of the social environment to fine-tune the experience-dependent plasticity of the social brain (Jurek and Neumann 2018). Oxtr expression and distribution, as opposed to circulating Oxt levels per se, may hence be of particular significance. As we described in the earlier section, Oxt influences reward-related behavior in different ways: while it reduces motivated behavior for palatable food, it modulates social rewards and attention-orienting responses to external social cues (Liu et al. 2020). It is possible that dysfunctional social attachment to the parental figure during the early development (MS or LN) alters the oxytocinergic system maturation such that the reward response to feeding prevails as opposed to the reward-response to social cues. Such paradigmatic model may be exploited not only for the study of obesity vulnerability, but also of psychiatric diseases such as eating disorders and addictions. However, the limitations related to the use of animal models for the study of such complex diseases should be acknowledged. Growth in an unpredictable environment where maternal care (feeding) is not constantly guaranteed, may result in a neurometabolic programming which aims at maximizing energy accrual and minimizing its waste. As a result, besides the enhanced reward value of food, the ability to store energy to face an adverse environment may be increased as well. Future researches, going in depth into the biological underpinnings of ELS-induced metabolic and eating behaviors anomalies, should be performed to shed light on novel, etiological mechanisms resposible for obesity and to provide novel preventive and/or therapeutic strategies.
Abces in the Oxt system may affect the adipose proliferative niches (hence, adipose tissue development) and may partly contribute to the distinctive fat mass distribution and susceptibility to obesity comorbidities described in males and females. Furthermore, while the effect of Oxt manipulation in early development has bink, M. R., A. F. van Deijk, V. M. Heine, M. H. Verheijen, and A. Korosi. 2019. 'The involvement of astrocytes in early-life adversity induced programming of theen widely investigated brain'[42], Glia, 67: 1637-53.
Akbarli, P.,ttle A. Gilani, O. Sosina, J. A. Kosmicki, L. Khrimian, Y. Y. Fang, T. Persaud, V. Garcia, D. Sun, A. Li, J. Mbatchou, A. E. Locke, C. Benner, N. Verweij, N. Lin, S. Hossain, K. Agostinucci, J. V. Pascale, E. Dirice, M. Dunn, Center Regeneron Genetics, E. H. R. Collaboration Discov, W. E. Kraus, S. H. Shah, Y. I. Chen, J. I. Rotter, D. J. Rader, O. Melander, C. D. Still, T. Mirshahi, D. J. Carey, J. Berumen-Campos, P. Kuri-Morales, J. Alegre-Diaz, J. M. Torres, J. R. Emberson, R. Collins, S. Balasubramanian, A. Hawes, M. Jones, B. Zambrowicz, A. J. Murphy, C. Paulding, G. Coppola, J. D. Overton, J. G. Reid, A. R. Shuldiner, M. Cantor, H. M. Kang, G. R. Abecasis, K. Karalis, A. N. Economides, J. Marchini, G. D. Yancopoulos, M. W. Sleeman, J. Altarejos, G. Della Gatta, R. Tapia-Conyer, M. L. Schwartzman, A. Baras, M. A. R. Ferreira,is known about the function of endogenous Oxt at such a time and L. A[23]. Lotta. 2021. 'Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity', Science, 373.
Andermverann,l M. L., and B. B. Lowell. 2017. 'Toward a Wiring Diagram Understanding of Appetite Control', Neuron, 95: 757-78.
Blines of researacz, S. J., N. A. Everett, and J. L. Cornish. 2020. 'The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy', Neurosci Biobehav Rev, 110: 114-32.
Bluher, M. 2019. 'Obesih have demonstraty: global epidemiology and pathogenesis', Nat Rev Endocrinol, 15: 288-98.
Bod howlby, J. 1969. Attachment and Loss (Basic Books).
Brpay, G. A., K. K. Kim, J. P. H. Wilding, and Federation World Obesity. 2017. 'Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation',-temporal Oxtr Obes Rev, 18: 715-23.
Carter, C. S. 2017. 'The Role of Oxytocin and Vasopressin in Attachment', Psychodyn Psychiatry, 45: 499-517.
Colleluori, G., J. Perugini, G. Barbatelli, and S. Cinti. 2021. 'Mammary gland adipocytes in lactation cycle, obesity and breast cancer', Rev Endocr Metab Disord, 22: 241-55.
Curley, J. P., and F. A. Cham varies in respagone. 2016. 'Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods'se to environmental cues, Front Neuroendocrinol, 40: 52-66.
de Lima, R. M. S., L. V. Dos Santos Bento, M. di Marcello Valladao Lugon, V. G. Barauna, A. S. Bittencourt, C. Dalmaz, and A. P. S. de Vasconcellos Bittencourt. 2020. 'Early life stress and the programming of eating behavior and anxiety: Sex-specific relationships with serotonergic activity and hypothalamic neuropeptides', Behav Brain Res, 379: 112399.
Ding, C., M. K. Lepecially during critical periow, and F. Magkos. 2019. 'Oxytocin in metabolic homeostasis: implications for obesity and diabetes management', Obes Rev, 20: 22-40.
EASO. 2020. "Obesits. It is widely Statistics." In, edited by European Association for the Study of Obesity.
Eller, O. C., E. M. Morris, J. P. Tccepted thyfault, and J. A. Christianson. 2020. 'Early life stress reduces voluntary exercise and its prevention of diet-induced obesity and metabolic dysfunction in mice',t Oxtr may be Physiol Behav, 223: 113000.
Ellis, B. J., A. J. Horn, C. S. Carter, IJzendoorn M. H. van, and M. J. Bakermans-Kranenburg. 2021. 'Dedevelopmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation',lasticity gene that Clin Psychol Rev, 86: 101985.
Espinoza, S. 2019. 'The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Completed.', Accessed 24/01/2022. https://clinicaltrials.gov/ct2/show/NCT03119610.
Feldman, R. 2017. 'The Neuts as a transducerobiology of Human Attachments',the Trends Cogn Sci, 21: 80-99.
Francis, D. D., F. C. Champagne, and M. J. Meaney. 2000. 'Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat', J Neuroendocrinol, 12: 1145-8.
Francis, D. D., L. J. Yocial environment to finetung, M. J. Meaney, and T. R. Insel. 2002. 'Naturally occurring differences in maternal care are associated with t the expression of oxytocin and vasopressin (V1a) receptors: gender differences', J Neuroendocrinol, 14: 349erience-53.
Garcia-Cacderpes, C., E. Balland, V. Prevot, S. Luquet, S. C. Woods, M. Koch, T. L. Horvath, C. X. Yi, J. A. Chowen, A. Verkhratsky, A. Araque, I. Bechmann, and M. H. Tschop. 2019. 'Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism', Nat Neurosci, 22: 7-14.
Geserick, M., M. Vogel, R. Gauscndent plasticity of the, T. Lipek, U. Spielau, E. Keller, R. Pfaffle, W. Kiess, and A. Korner. 2018. 'Acceleration of BMI in Early Childhood and Risk of Sustained Obesity', N Engl J Med, 379: 1303-12.
Giosocial brdano,in A[33].; Nisoli, E. 2018. 'Neuroendocrinology of Energy Balance'Therefore, Obesity.
Goodwill, H. L., G. Manzano-Nieves, M. Gallo, H. I. Lee, E. Oyerinde, T. Serre, and K. G. Baxth. 2019. 'Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model',r expression and distribution, as opposed Neuropsychopharmacology, 44: 711-20.
Jurek, B., and I. D. Neumann. 2018. 'The Oxyto cin Receptor: From Intracellular Signaling to Behavior', Physiol Rev, 98: 1805-908.
Kim, S., S. Kwok, L. C. Mayrculating Oxt levels, M. N. Potenza, H. J. V. Rutherford, and L. Strathearn. 2017. 'Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways', Ann N Y Acad Sci, 1394: 74-91.
Kra per se, may be of particuse, S., C. Boeck, A. M. Gumpp, E. Rottler, K. Schury, A. Karabatsiakis, A. Buchheim, H. Gundel, I. T. Kolassa, and C. Waller. 2018. 'Child Maltreatment Is Associated with a Reduction of the Oxytocin Receptor in Peripheral Blood Mononuclear Cells', Front Psychol, 9: 173.
Lar significance. As noted above, Oxt influences reward-relawson, E. A. 2017. 'The effects of oxytocin on eatinged behaviour and metabolism in humans', Nat Rev Endocrinol, 13: 700-09.
———. 2022. 'A Ranrs in domized, Double-blind, Placebo-controlled Study to Evaluate the Effects of Repeat Doses of Intranasal Oxytocin in Obese Adults. Completed.'. https://clinicaltrials.gov/ct2/show/NCT03043053.
Lafferent ways: wson, E. A., P. K. Olszewskhi, A. Weller, and J. E. Blevins. 2020. 'The role of oxytocin in regulation of appetitivele it reduces motivated behaviour, body weight and glucose homeostasis', J Neuroendocrinol, 32: r toward palatable12805.
Leng, G., R. A. H. Adan, M. Belfot, J. M. Brunstrom, K. de Graaf, S. L. Dickson, T. Hare, S. Maier, J. Menzies, H. Preissl, L. A. Reisch, P. J. Rogers, and P. A. M. Smeets. 2017. 'The determinants of food choice', Proc Nutr Soc, 76: 316-27.
Leng, G., C. Caqod, it moduinelau, and N. Sabatier. 2005. 'Regulation of oxytocin secretion',tes Vitam Horm, 71: 27-58.
Leng, Gareth. 2018. The Heart of the Brain: The Hypothalamus and Its Hormones (The MIT Press).
Liao, P. Y., Y. M. Chciu, J. H. Yu, and S. K. Chen. 2020. 'Mapping Central Projection of Oxytocin Neurons in Unmated Mice Using Cre and Alkaline Phosphatase Reporter', Front Neuroanat, 14: 559402.
Liu, C. M., T. M. Hsu, A. N. Suarez, K. S. Subramaniaal rewards and atten, R. A. Fatemi, A. M. Cortella, E. E. Noble, M. F. Roitman, and S. E. Kanoski. 2020. 'Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine respion-orienting responses to food-predictive cues in male rats', Horm Behav, 126: 104855.
Lexternal socke, A. E., B. Kahiali, S. I. Berndt, A. E. Justice, T. H. Pers, cues F[55]. R. Day, C. Powell, S. VedanItam, M. L. Buchkovich, J. Yang, D. C. Croteau-Chonka, T. Esko, T. Fall, T. Ferreira, S. Gustafsson, Z. Kutalik, J. Luan, R. Magi, J. C. Randall, T. W. Winkler, A. R. Wood, T. Workalemahu, J. D. Faul, J. A. Smith, J. H. Zhao, W. Zhao, J. Chen, R. Fehrmann, A. K. Hedman, J. Karjalainen, E. M. Schmidt, D. Absher, N. Amin, D. Anderson, M. Beekman, J. L. Bolton, J. L. Bragg-Gresham, S. Buyske, A. Demirkan, G. Deng, G. B. Ehret, B. Feenstra, M. F. Feitosa, K. Fischer, A. Goel, J. Gong, A. U. Jackson, S. Kanoni, M. E. Kleber, K. Kristiansson, U. Lim, V. Lotay, M. Mangino, I. M. Leach, C. Medina-Gomez, S. E. Medland, M. A. Nalls, C. D. Palmer, D. Pasko, S. Pechlivanis, M. J. Peters, I. Prokopenko, D. Shungin, A. Stancakova, R. J. Strawbridge, Y. J. Sung, T. Tanaka, A. Teumer, S. Trompet, S. W. van der Laan, J. van Setten, J. V. Van Vliet-Ostaptchouk, Z. Wang, L. Yengo, W. Zhang, A. Isaacs, E. Albrecht, J. Arnlov, G. M. Arscott, A. P. Attwood, S. Bandinelli, A. Barrett, I. N. Bas, C. Bellis, A. J. Bennett, C. Berne, R. Blagieva, M. Bluher, S. Bohringer, L. L. Bonnycastle, Y. Bottcher, H. A. Boyd, M. Bruinenberg, I. H. Caspersen, Y. I. Chen, R. Clarke, E. W. Daw, A. J. M. de Craen, G. Delgado, M. Dimitriou, A. S. F. Doney, N. Eklund, K. Estrada, E. Eury, L. Folkersen, R. M. Fraser, M. E. Garcia, F. Geller, V. Giedraitis, B. Gigante, A. S. Go, A. Golay, A. H. Goodall, S. D. Gordon, M. Gorski, H. J. Grabe, H. Grallert, T. B. Grammer, J. Grassler, H. Gronberg, C. J. Groves, G. Gusto, J. Haessler, P. Hall, T. Haller, G. Hallmans, C. A. Hartman, M. Hassinen, C. Hayward, N. L. Heard-Costa, Q. Helmer, C. Hengstenberg, O. Holmen, J. J. Hottenga, A. L. James, J. M. Jeff, A. Johansson, J. Jolley, T. Juliusdottir, L. Kinnunen, W. Koenig, M. Koskenvuo, W. Kratzer, J. Laitinen, C. Lamina, K. Leander, N. R. Lee, P. Lichtner, L. Lind, J. Lindstrom, K. S. Lo, S. Lobbens, R. Lorbeer, Y. Lu, F. Mach, P. K. E. Magnusson, A. Mahajan, W. L. McArdle, S. McLachlan, C. Menni, S. Merger, E. Mihailov, L. Milani, A. Moayyeri, K. L. Monda, M. A. Morken, A. Mulas, G. Muller, M. Muller-Nurasyid, A. W. Musk, R. Nagaraja, M. M. Nothen, I. M. Nolte, S. Pilz, N. W. Rayner, F. Renstrom, R. Rettig, J. S. Ried, S. Ripke, N. R. Robertson, L. M. Rose, S. Sanna, H. Scharnagl, S. Scholtens, F. R. Schumacher, W. R. Scott, T. Seufferlein, J. Shi, A. V. Smith, J. Smolonska, A. V. Stanton, V. Steinthorsdottir, K. Stirrups, H. M. Stringham, J. Sundstrom, M. A. Swertz, A. J. Swift, A. C. Syvanen, S. T. Tan, B. O. Tayo, B. Thorand, G. Thorleifsson, J. P. Tyrer, H. W. Uh, L. Vandenput, F. C. Verhulst, S. H. Vermeulen, N. Verweij, J. M. Vonk, L. L. Waite, H. R. Warren, D. Waterworth, M. N. Weedon, L. R. Wilkens, C. Willenborg, T. Wilsgaard, M. K. Wojczynski, A. Wong, A. F. Wright, Q. Zhang, Study LifeLines Cohort, E. P. Brennan, M. Choi, Z. Dastani, A. W. Drong, P. Eriksson, A. Franco-Cereceda, J. R. Gadin, A. G. Gharavi, M. E. Goddard, R. E. Handsaker, J. Huang, F. Karpe, S. Kathiresan, S. Keildson, K. Kiryluk, M. Kubo, J. Y. Lee, L. Liang, R. P. Lifton, B. Ma, S. A. McCarroll, A. J. McKnight, J. L. Min, M. F. Moffatt, G. W. Montgomery, J. M. Murabito, G. Nicholson, D. R. Nyholt, Y. Okada, J. R. B. Perry, R. Dorajoo, E. Reinmaa, R. M. Salem, N. Sandholm, R. A. Scott, L. Stolk, A. Takahashi, T. Tanaka, F. M. van 't Hooft, A. A. E. Vinkhuyzen, H. J. Westra, W. Zheng, K. T. Zondervan, A. DIPOGen Consortium, Agen-Bmi Working Group, C. ARDIOGRAMplusC4D Consortium, C. KDGen Consortium, Glgc, Icbp, Magic Investigators, Ther Consortium Mu, M. IGen Consortium, Page Consortium, Consortium ReproGen, Genie Consortium, Consortium International Endogene, A. C. Heath, D. Arveiler, S. J. L. Bakker, J. Beilby, R. N. Bergman, J. Blangero, P. Bovet, H. Campbell, M. J. Caulfield, G. Cesana, A. Chakravarti, D. I. Chasman, P. S. Chines, F. S. Collins, D. C. Crawford, L. A. Cupples, D. Cusi, J. Danesh, U. de Faire, H. M. den Ruijter, A. F. Dominiczak, R. Erbel, J. Erdmann, J. G. Eriksson, M. Farrall, S. B. Felix, E. Ferrannini, J. Ferrieres, I. Ford, N. G. Forouhi, T. Forrester, O. H. Franco, R. T. Gansevoort, P. V. Gejman, C. Gieger, O. Gottesman, V. Gudnason, U. Gyllensten, A. S. Hall, T. B. Harris, A. T. Hattersley, A. A. Hicks, L. A. Hindorff, A. D. Hingorani, A. Hofman, G. Homuth, G. K. Hovingh, S. E. Humphries, S. C. Hunt, E. Hypponen, T. Illig, K. B. Jacobs, M. R. Jarvelin, K. H. Jockel, B. Johansen, P. Jousilahti, J. W. Jukema, A. M. Jula, J. Kaprio, J. J. P. Kastelein, S. M. Keinanen-Kiukaanniemi, L. A. Kiemeney, P. Knekt, J. S. Kooner, C. Kooperberg, P. Kovacs, A. T. Kraja, M. Kumari, J. Kuusisto, T. A. Lakka, C. Langenberg, L. L. Marchand, T. Lehtimaki, V. Lyssenko, S. Mannisto, A. Marette, T. C. Matise, C. A. McKenzie, B. McKnight, F. L. Moll, A. D. Morris, A. P. Morris, J. C. Murray, M. Nelis, C. Ohlsson, A. J. Oldehinkel, K. K. Ong, P. A. F. Madden, G. Pasterkamp, J. F. Peden, A. Peters, D. S. Postma, P. P. Pramstaller, J. F. Price, L. Qi, O. T. Raitakari, T. Rankinen, D. C. Rao, T. K. Rice, P. M. Ridker, J. D. Rioux, M. D. Ritchie, I. Rudan, V. Salomaa, N. J. Samani, J. Saramies, M. A. Sarzynski, H. Schunkert, P. E. H. Schwarz, P. Sever, A. R. Shuldiner, J. Sinisalo, R. P. Stolk, K. Strauch, A. Tonjes, D. A. Tregouet, A. Tremblay, E. Tremoli, J. Virtamo, M. C. Vohl, U. Volker, G. Waeber, G. Willemsen, J. C. Witteman, M. C. Zillikens, L. S. Adair, P. Amouyel, F. W. Asselbergs, T. L. Assimes, M. Bochud, B. O. Boehm, E. Boerwinkle, S. R. Bornstein, E. P. Bottinger, C. Bouchard, S. Cauchi, J. C. Chambers, S. J. Chanock, R. S. Cooper, P. I. W. de Bakker, G. Dedoussis, L. Ferrucci, P. W. Franks, P. Froguel, L. C. Groop, C. A. Haiman, A. Hamsten, J. Hui, D. J. Hunter, K. Hveem, R. C. Kaplan, M. Kivimaki, D. Kuh, M. Laakso, Y. Liu, N. G. Martin, W. Marz, M. Melbye, A. Metspalu, S. Moebus, P. B. Munroe, I. Njolstad, B. A. Oostra, C. N. A. Palmer, N. L. Pedersen, M. Perola, L. Perusse, U. Peters, C. Power, T. Quertermous, R. Rauramaa, F. Rivadeneira, T. E. Saaristo, D. Saleheen, N. Sattar, E. E. Schadt, D. Schlessinger, P. E. Slagboom, H. Snieder, T. D. Spector, U. Thorsteinsdottir, M. Stumvoll, J. Tuomilehto, A. G. Uitterlinden, M. Uusitupa, P. van der Harst, M. Walker, H. Wallaschofski, N. J. Wareham, H. Watkins, D. R. Weir, H. E. Wichmann, J. F. Wilson, P. Zanen, I. B. Borecki, P. Deloukas, C. S. Fox, I. M. Heid, J. R. O'Connell, D. P. Strachan, K. Stefansson, C. M. van Duijn, G. R. Abecasis, L. Franke, T. M. Frayling, M. I. McCarthy, P. M. Visscher, A. Scherag, C. J. Willer, M. Boehnke, K. L. Mohlke, C. M. Lindgren, J. S. Beckmann, I. Barroso, K. E. North, E. Ingelsson, J. N. Hirschhorn, R. J. F. Loos, and E. K. Speliotes. 2015. 'Genetic studies of body mass index yield new insights for obesity biology', Nature, 518: 197-206.
Maniam, J., C. P. Antoniadis, K. W. Wang, and M. J. Mor is reasonable to hypothesize that dysfunctional social attachment to the parental figure during early development (MS or LN) alters the oxytocinergic system maturation such that the reward response to feeding prevails on the reward response to social cues. This paradigmatic model may be employed not only to study obesity vulnerability, but also psychiatric diseases, such as eating disorders, depression, and addiction. Indeed, the limitations related to the use of animal models for the study of such complex diseases should be acknowledged. Growth in an unpredictable environment, wheris. 2015. 'Early Life Stress Induced by Limited Nesting Material Produces Metabolic Resilience in Response to a High-Fat and High-Sugar Diet in Male Rats', Front Endocrinol (Lausanne),maternal 6: 138.
McCook, O., N. Denoix, P. Radermacher, C. Waller, and T. Merz. 2021. 'H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease', J Clin Med, 10.
McCormack, S. 2022. 'Inre (feeding) is notranasal Oxytocin to Promote Weight Loss in Children, Adolescents, and Adults With Brain Tumors and Hypothalamic Obesity Syndrome. Recruiting.', NIH, Accessed 24/01/2022. https://clinicaltrials.gov/ct2/show/NCT02849743.
Miller, A. L., and J. C. Lconstantly ensumeng. 2018. 'Pathways of Association from Stress to Obesity in Early Childhood'd, Obesity (Silver Spring), 26: 1117-24.
Miller, G. E., E. Chen, mand K. J. Parker. 2011. 'Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms', Psychol Bull, 137: 959-97.
Molet, J., P. M. Maras, S. Avishai-Eliner, and T. Z. Baram. 2014. 'Nat result in a neuralistic rodent models of chronic early-life stress', Dev Psychobiol, 56: 1675-88.
Moreno-Mendez, E.; Quintero-Fmetabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M. 2020. 'Early-lifelic programming of adipose tissue', Nutrition Research Reviews: 1-16.
Myers, M. G., Jr., A. H. Affinati, N. Ricthardson, and M. W. Schwartz. 2021. 'Central nervous system regulation of organismal maximizes energy and glucose homeostasis', Nat Metab, 3: 737-50.
Nelson, C. A., 3ccrd, and L. J. Gabard-Durnam. 2020. 'Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment', Trends Neurosci, 43: 133-43.
Noval and minimick, A. M., M. L. Lzevandowski, L. E. Laumann, N. S. Philip, L. H. Price, and A. R. Tyrka. 2018. 'The effects of early life stress on reward processing', J Psychiatr Res, 101: 80-103.
Pas its wasternain, L., E. Martisova, F. I. Milagro, M. J. Ramirez, J. A. Martinez, and J. Campion. 2012. 'Postnatal maternal separation modifi As a result, besides the response to an obesogenic diet in adulthood in rats', Dis Model Mech, 5: 691-7.
Ricenhanced re, C. J., C. A. Swandman, M. R. Lenjavi, and T. Z. Baram. 2008. 'A novel mouse model for acute and long-lasting consequences of early life stress', Endocrinology, 149: 4892-900.
Ruiz, R., A. Roque, E. Pineda, P. Licona-Limon, J. Jose Valdez-Alarcon, and N. Lajud. 2018. 'Earlrd value of food, the ability life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk', Psychoneuroendocrinology, 96: 203-11.
Ro store energyu, V., S. B. Yoo, D. W. Kang, J. H. Lee, and J. W. Jahng. 2009. 'Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation',to cope with an adverse Brain Res, 1295: 127-34.
Science, UK Goverironment Office for. 2007. "Tackling Obesities: Future Choices - Project Report." In, edited by Foresight Programme.
Szczepanska-Samay also be increasedowska, E., A. Wsol, A. Cudnoch-Jedrzejewska, and T. Zera. 2021. 'Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation', Deeper investigation Int J Mol Sci, 22.
Tsuda, M. C., N. Yamaguchi, and S. Ogawa. 2011. 'Early liofe stress disrupts peripubertal development of aggression in male mice', Neuroreport, 22: 259-63.
Veenemthe biologica, A. H., R. Bredewold, and I. D. Neumann. 2007. 'Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity', Psychoneuroendocrinology, 32: 437-50.
Ward, Z. J., S. N. Bl underpinnings of ELS-induceich, A. L. Cradock, J. L. Barrett, C. M. Giles, C. Flax, M. W. Long, and S. L. Gortmaker. 2019. 'Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity', N Engl J Med, 381: 2440-50.
W metabolic and eaterson, M. J., and T. L. Horvath. 2015. 'Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding', Cell Metab, 22: 962-70.
Wei, F., W. Li, B. Ma, X. Deng, L. Zhang, L. Zhao, T. Zheng, and Y. Jing. 2021. 'Experiences affect social ng behaviors via altering neuronal morphology and oxytocin system', Psychoneuroendocrinology, 129: 105247.
WHO. 2018. 'Obesity Report', Accessed Jabnormanuary 25th
———. 2020. 'WHO European Childhood Obesity Surveillance Initiative (COSI)'. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/who-european-childhood-obesity-surveillance-initiative-cosi.
Wigger, D. C., N. Groger, A. Lesies ise, S. Krause, T. Merz, H. Gundel, K. Braun, O. McCook, P. Radermacher, J. Bock, and C. Waller. 2020. 'Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice', Oxid Med Cell Longev, 2020: 4309605.
Wilwarranted to characterize unexpliamson, E. J., A. J. Walker, K. Bhaskaran, S. Bacon, C. Bates, C. E. Morton, H. J. Curtis, A. Mehrkar, D. Evans, P. Inglesby, J. Cockburn, H. I. McDonald, B. MacKenna, L. Tomlinson, I. J. Douglas, C. T. Rentsch, R. Mathur, A. Y. S. Wong, R. Grieve, D. Harrison, H. Forbes, A. Schultze, R. Croker, J. Parry, F. Hester, S. Harper, R. Perera, S. J. W. Evans, L. Smeeth, and B. Goldacre. 2020. 'Factors associated with COVID-19-related death using OpenSAFELY', Nature, 584: 430-36.
Winnicott, D. W. 1957. Mother and child: A primer of first relationships (Bared mechanisms responsic Books).
Yam, K. Y., E. F. G. Naninck, M. R. Abbink, S. E. la Fleur, L. Schipper, J. C. van den Beukel, A. Grefhorst, A. Oosting, E. M. van der Beek, P. J. Lucassen, and A. Korosi. 2017. 'Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice', Psychoneuroendocrinology, 77: 186-95.
Yamam for obesity and to identify noto, Y., B. S. Cushing, K. M. Kramver, P. D. Epperson, G. E. Hoffman, and C. S. Carter. 2004. 'Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner', Neuroscience, 125: 947-55.
Zhang, H., C. Wu, Q. Chen, X. Chen, Z. Xu, J. Wu, and D. Cai. 2013. 'Treal preventive and/or therapeutic stratment of obesity and diabetes using oxytocin or analogs in patients and mouse models', PLoS One, 8: e61477gies.
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298.
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450.
- EASO. Eauropean Association for the Study of Obesity. Obesity Statistics. 2020. Available online: https://www.karger.com/Article/FullText/508082 (accessed on 24 January 2022).
- WHO. WHO European Childhood Obesity Surveillance Initiative (COSI). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/who-european-childhood-obesity-surveillance-initiative-cosi (accessed on 24 January 2022).
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Korner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312.
- WHO. Obesity Report. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 January 2021).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity, F. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723.
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255.
- US Government Office for Science. Tackling Obesities: Future Choices—Project Report. 2007. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/287937/07-1184x-tackling-obesities-future-choices-report.pdf (accessed on 7 February 2022).
- Giordano, A.; Nisoli, E. Neuroendocrinology of Energy Balance. Obesity, Endocrinology. 2018. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-319-47685-8_4-1?noAccess=true (accessed on 10 February 2022).
- Leng, G.; Adan, R.A.H.; Belot, M.; Brunstrom, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The determinants of food choice. Proc. Nutr. Soc. 2017, 76, 316–327.
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778.
- Myers, M.G., Jr.; Affinati, A.H.; Richardson, N.; Schwartz, M.W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 2021, 3, 737–750.
- Leng, G. The Heart of the Brain: The Hypothalamus and Its Hormones; The MIT Press: Cambridge, MA, USA, 2018.
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970.
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206.
- Akbari, P.; Gilani, A.; Sosina, O.; Kosmicki, J.A.; Khrimian, L.; Fang, Y.Y.; Persaud, T.; Garcia, V.; Sun, D.; Li, A.; et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 2021, 373, 8683.
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259.
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997.
- Miller, A.L.; Lumeng, J.C. Pathways of Association from Stress to Obesity in Early Childhood. Obesity 2018, 26, 1117–1124.
- Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn. Psychiatry 2017, 45, 499–517.
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805.
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132.
- Bowlby, J. Attachment and Loss, 2nd ed.; Basic Books: New York, NY, USA, 1969.
- Winnicott, D.W. Mother and Child: A Primer of First Relationships; Basic Books: New York, NY, USA, 1957.
- Nelson, C.A., 3rd; Gabard-Durnam, L.J. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020, 43, 133–143.
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900.
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688.
- Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66.
- Garcia-Caceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14.
- Abbink, M.R.; van Deijk, A.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia 2019, 67, 1637–1653.
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436.
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908.
- Feldman, R. The Neurobiology of Human Attachments. Trends Cogn. Sci. 2017, 21, 80–99.
- Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.V.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91.
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709.
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40.
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477.
- McCormack, S. Intranasal Oxytocin to Promote Weight Loss in Children, Adolescents, and Adults with Brain Tumors and Hypothalamic Obesity Syndrome. Recruiting. Available online: https://clinicaltrials.gov/ct2/show/NCT02849743 (accessed on 24 January 2022).
- Lawson, E.A. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effects of Repeat Doses of Intranasal Oxytocin in Obese Adults. Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03043053 (accessed on 24 January 2022).
- Espinoza, S. The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03119610 (accessed on 24 January 2022).
- Yamamoto, Y.; Cushing, B.S.; Kramer, K.M.; Epperson, P.D.; Hoffman, G.E.; Carter, C.S. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 2004, 125, 947–955.
- Leng, G.; Caquineau, C.; Sabatier, N. Regulation of oxytocin secretion. Vitam. Horm. 2005, 71, 27–58.
- Liao, P.Y.; Chiu, Y.M.; Yu, J.H.; Chen, S.K. Mapping Central Projection of Oxytocin Neurons in Unmated Mice Using Cre and Alkaline Phosphatase Reporter. Front. Neuroanat. 2020, 14, 559402.
- Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000, 12, 1145–1148.
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353.
- Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259–263.
- Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450.
- Wei, F.; Li, W.; Ma, B.; Deng, X.; Zhang, L.; Zhao, L.; Zheng, T.; Jing, Y. Experiences affect social behaviors via altering neuronal morphology and oxytocin system. Psychoneuroendocrinology 2021, 129, 105247.
- Ellis, B.J.; Horn, A.J.; Carter, C.S.; van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Developmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 2021, 86, 101985.
- Krause, S.; Boeck, C.; Gumpp, A.M.; Rottler, E.; Schury, K.; Karabatsiakis, A.; Buchheim, A.; Gundel, H.; Kolassa, I.T.; Waller, C. Child Maltreatment Is Associated with a Reduction of the Oxytocin Receptor in Peripheral Blood Mononuclear Cells. Front. Psychol. 2018, 9, 173.
- Wigger, D.C.; Groger, N.; Lesse, A.; Krause, S.; Merz, T.; Gundel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice. Oxid. Med. Cell Longev. 2020, 2020, 4309605.
- McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J. Clin. Med. 2021, 10, 3484.
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Zera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465.
- Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855.
