Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 5 by Florian Zikeli and Version 4 by Camila Xu.
Polyurethanes (PUs) present an important class of polymers due to outstanding mechanical, chemical and physical properties. Thus, they find application in many industrial sectors in the form of flexible or rigid foams, coatings, adhesives, elastomers, thermoplasts or thermosets. Modern PU coating applications include self-healing coating films that can also be applied to rather rough surfaces, such as wood.
- Wood coating
- Wood color
- Contact angle
- SEM
- FTIR Mapping
- Beech wood
- FTIR
1. Introduction
Wood materials and wood-based products are increasingly considered the materials of the future, fully in line with modern concepts of a bio-circular economy [1][2]. However, the intrinsic biodegradability of wood is the most important weakness, which limits a wider end use, and thus the improvement of wood durability is still one of the major challenges [3][4]. This challenge can be tackled by different means, such as thermo-treatments [5][6] or fossil-based and natural wood coatings with protective or biocidal functions [7][8][9][10].
A definition of “coating material” is given by the European Standard EN 971-1 [11]: “A product, in liquid, in paste or powder form, that, when applied to a substrate, forms a film possessing protective, decorative and/or other specific properties”. Various types of organic substances have been used in the preparation of coating materials for wood. In ancient times, only natural products such as oils or resins were available [12][13]. Later on, the application of thermal or chemical modifications improved the performance of natural products. A continuously developing chemical industry in the first half of the 20th century produced a wide range of synthetic coating films, such as alkyds, polyurethanes and polyesters, which were successfully applied in many fields, including wood technology [14][15][16][17][18]. For the modern coating industry, polyurethanes (PUs) present an important class of polymers due to outstanding mechanical, chemical and physical properties. Thus, they find application in many industrial sectors in the form of flexible or rigid foams, coatings, adhesives, elastomers, thermoplasts or thermosets [8][19]. Modern PU coating applications include self-healing coating films that can also be applied to rather rough surfaces, such as wood [6]. Polyurethanes are available as solvent or water-borne formulations, and polymer curing may be brought about at ambient or elevated temperatures. One of the major issues of polyurethanes is their fossil-based origin and the toxicity of reactants and pre-cursors such as isocyanates, which act as a modifier or crosslinking agent, rather than the main film former. Conventional PUs are usually synthesized by a polyaddition reaction between polyols and poly-isocyanates. These two, when reacted together, form the urethane linkages observed in a crosslinked polymer. Recently, several attempts were reported to substitute fossil-based building blocks of PU coatings with bio-based compounds because the versatility in chemistry and processing enables wide opportunities for introducing new raw materials into various PU products [20]. Recent research utilizes castor oil for the preparation of a hyperbranched polyol that was further used for a bio-based PU coating with self-cleaning abilities [21]. Besides vegetable oils (i.e., from soybean) or starch, which have been extensively applied as natural and renewable polyol sources for PUs, lignin has emerged as a promising alternative polyol source for application in resins, adhesives and foams [22][23]. After cellulose, lignin is the second most abundant natural polymer, containing a broad range and high contents of chemical functional groups such as hydroxyls, carbonyls and carboxyls [24], which allow for its utilization as an alternative for polymer development, especially as a substitute for petroleum-based polyols in polyurethane systems. Scientific contributions regarding the substitution of polyols by lignin in polyurethanes involve, in general, kraft lignins [25], while the performance of other technical lignins, i.e., Organosolv lignins, has not yet been evaluated.
2. Color Measurements
Figure 1 shows the beech wood samples coated with the different PU formulations, and their respective color coordinates are listed in Table 1.


Figure 1. Beech wood samples before (a) and after coating application (b–e). (a) W-R, (b) WPU-R, (c) WPU-3:1, (d) WPU-1:1 and (e) WPU-1:3.
Table 1. Color coordinates based on CIELAB color space of wooden samples before coating application (W-R) and after coating application. ΔE* was calculated using the difference between uncoated (W-R) and coated wood samples. Statistical differences are reported as: *** p < 0.001, ** p < 0.01 and * p < 0.05.
| Before Coating (W-R) | After Coating | |||||
|---|---|---|---|---|---|---|
| Thickness | (μm) |
SC (%) |
||||
| WPU-R | 5.2 ± 1.7 | 28.2 ± 20.6 | 57.1 1 | |||
| WPU-3:1 | 0.6 ± 1.7 *** | 25.9 ± 7.0 | 45.1 2 | |||
| WPU-1:1 | 0.8 ± 1.6 *** | 28.1 ± 7.1 | 47.1 3 | |||
| WPU-1:3 | 0.7 ± 1.3 *** | 27.8 ± 9.2 | 51.0 4 | |||
4. Optical Contact Angle
In Table 3, the contact angle (CA) values are reported. As expected, the commercial coating had the highest CAs (Figure 2a), while higher lignin contents in the prepared PU coating led to lower CAs (Figure 2b). The CA of W-R changes strongly within the first 10 s after droplet deposition, while for WPU-R it remains stable, meaning that the water drop was adsorbed in the first case while in the latter the film is waterproof, as expected. In the coatings of the other PU formulations, the differences are not pronounced, which is why they are considered as waterproof as well, even if the CAs of the prepared PU formulations are lower than WPU-R. In the case of WPU-1:3, the water drop was completely absorbed within 10 min, as occurred with W-R. Standard deviations of prepared PU formulations are a little high, which might depend on the irregularity of the substrate, which in turn depends on the coating application. In fact, during brush application, PU formulations dried as they were applied, making the surface not flat. To summarize, it seems that the hydrophobicity of the prepared PU formulations decreased when lignin content increased. Griffini et al. [27] address this behavior to the presence of hydroxyl groups in lignin, which are higher for increasing lignin content in PU materials, leading to lower CAs. Another reason for this behavior could be explained by the cracks visible on the prepared PU surfaces (Figure 3a–c), which let the water pass through the coating film. CA values are significant only when the lignin concentration is the highest.
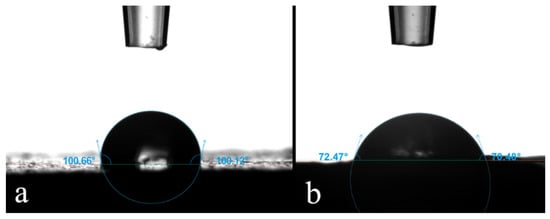
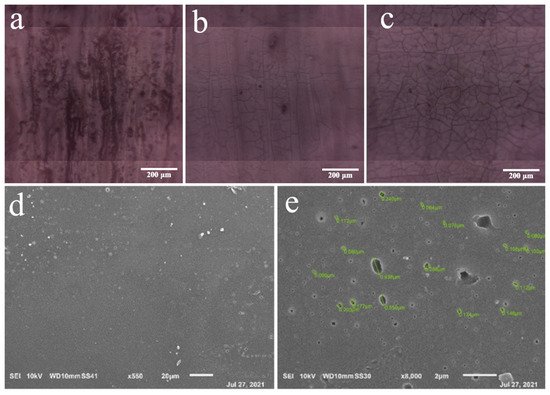

Figure 2. Two examples of CA measurement after drop release. In (a) a WPU-R sample, with a high CA, and in (b) a WPU-1:3 sample, with a low CA. Blue angles indicate how the instrument extrapolates the data from the images.

Figure 3. Pictures of measurement areas: (a) WPU-3:1, (b) WPU-1:1 and (c) WPU-1:3. SEM images of PU-3:1 (d,e).
Table 3. Contact angle (CA) measured for different prepared PU formulations. Values of CA after waterdrop deposition (CA-T0), CA after 10 s (CA-T10) and CA after 10 min (600 s) after waterdrop deposition (CA-T600) are reported. Statistical differences are reported as: *** p < 0.001, ** p < 0.01 and * p < 0.05.
| CA-T0 (°) |
|||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | ΔE* | |
| WPU-R | 85.8 ± 3.8 | 6.4 ± 2.2 | 19.8 ± 2.0 | 71.8 ± 2.0 *** | 11.3 ± 1.1 | 31.4 ± 2.6 | 19.5 ± 4.9 *** |
| WPU-3:1 | 85.6 ± 6.7 | 6.0 ± 2.4 | 20.3 ± 2.6 | 37.6 ± 5.3 *** | 26.4 ± 2.6 *** | 30.0 ± 5.5 | 54.7 ± 7.6 *** |
| WPU-1:1 | 86.2 ± 3.5 | 5.9 ± 2.0 | 21.3 ± 2.0 | 21.0 ± 4.3 *** | 21.1 ± 4.2 *** | 11.4 ± 5.8 *** | 68.7 ± 5.7 *** |
| WPU-1:3 | 90.1 ± 2.4 | 4.0 ± 1.8 | 19.7 ± 2.4 | 10.8 ± 2.5 *** | 7.8 ± 5.7 | 0.3 ± 1.0 *** | 82.3 ± 3.9 *** |
| Mean W-R | 86.9 ± 7.3 | 5.6 ± 2.9 | 20.3 ± 3.0 |
Virgin wood (W-R) color coordinates are quite homogeneous, but after coating application, the color strongly changes. Commercial coating (WPU-R) is transparent, so the color variations compared to raw solid wood are the lowest among the other PU formulations, and wood structural elements such as rays can still be identified (Figure 1a,b). In all cases, L* is lower, which means a darker color and less transparent coating, as seen in Figure 1c–e. The coordinate a* increases for all coatings, meaning a redder color than virgin wood. For the CIELAB coordinate b*, the situation is different. In WPU-R and WPU-3:1, b* increases, rendering the color more yellow, while in WPU-1:1 and WPU-1:3, b* changes the color to a bluer tone. This could be explained by the fact that in the first two samples (Figure 1a,b), wood underneath the coating is still visible, while the last two provide a less transparent coating (Figure 1c,d), where lignin covers the surface the most. Additionally, Jusic et al. [7] and Klein et al. [26] found a gradual color change at increasing lignin content. Changing color due to all formulations is always statistically significant (ΔE*).
3. Weight, Coating Film Thickness and Solid Content
In Table 2, the measured sample weights and their respective weight gains are reported as well as the determined solid contents (SC). Weight gain was much lower for the prepared PU coatings compared to the commercial reference (WPU-R), and the difference is statistically significant. The decrease of weight gain in increasing lignin concentration is consistent with the low values of SC for the prepared PU formulations, which could be explained by the presence of other additives in the commercial products increasing its SC. Regarding the prepared formulations, an increasing lignin content led to higher SC. The coating thicknesses of the three prepared formulations are quite similar (Table 2). It is noteworthy that WPU-R shows a very high standard deviation, which might be due to higher viscosity and the presence of additives, as can be deduced by the SC.
Table 2. Weight of the samples before and after application of the different coating formulations and respective weight gain, solid content (SC) of the pure formulations (1 PU-R, 2 PU-3:1, 3 PU-1:1, 4 PU-1:3). Statistical differences are reported as: *** p < 0.001, ** p < 0.01 and * p < 0.05.
| Weight Gain (%) |
CA-T10 (°) |
CA-T600 (°) |
|
|---|---|---|---|
| W-R | 93.9 ± 4.0 | 76.6 ± 5.9 | - |
| WPU-R | 89.5 ± 6.1 | 89.1 ± 6.2 | 79.9 ± 6.6 |
| WPU-3:1 | 82.0 ± 8.2 | 79.7 ± 7.8 | 69.1 ± 9.6 |
| WPU-1:1 | 79.8 ± 8.1 | 77.3 ± 8.5 | 54.7 ± 10.7 ** |
| WPU-1:3 | 79.0 ± 7.7 | 70.4 ± 5.4 ** | - |
Figure 2 shows how the instrument extrapolates CA values by contrast between the white background and the dark droplet.
5. FTIR Microscopy and Scanning Electron Microscopy (SEM)
The pictures of the measurement zones of the FTIR mapping of the three PU formulations were captured using the multi-channel Jasco infrared microscope IRT-7000 and a Cassegrain ×16 objective. These pictures show that with the increasing lignin concentration, changes in the morphology of the material occur, causing the formation of cracks in the coating. Hence, in the image of the sample WPU-3:1, the coating surface is less cracked (Figure 3a) than WPU-1:1 (Figure 3b) and WPU-1:3 (Figure 3c). This condition was confirmed by the acquisition of scanning electron microscope (SEM) images, where a smooth and homogeneous structure (Figure 3d) was observed with the presence of a few porosities with an average diameter of 0.25 µm (Figure 3e) and a minimal presence of lignin aggregates. In contrast to other works in the literature, where significant lignin aggregation was observed using SEM [28], the PU formulations prepared in this work were rather free from lignin aggregates, as illustrated in Figure 3d,e.
6. FTIR Spectroscopy and Imaging
In order to investigate the chemistry behind the preparation of the PU formulations, FTIR analysis was performed on samples of the three different PU formulations after both spontaneous (room temperature) and temperature-induced (60 °C for 72 h) crosslinking. The formation of urethane bonds was evaluated by studying the respective IR bands of the specific involved functional groups. The absorption band at 2270 cm−1 is associated to the stretching modes of the unreacted isocyanate NCO groups in the three different formulations (Figure 4a). The signal intensity of this band depends on the presence of excessive NCO groups after the formation of urethane bonds in the respective formulations. The disappearance of the isocyanate band in the sample PU-1:3 indicates a rather complete reaction between NCO and lignin OH groups, while the more intense absorption band in the spectra of the samples PU-3:1 and PU-1:1 reveals residual unreacted isocyanate in the respective formulations.
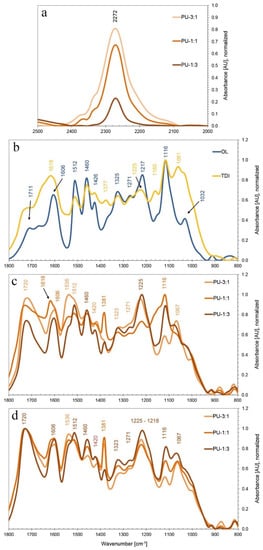

Figure 4. (a) Absorbance band of free isocyanate groups in the three different prepared PU formulations PU-3:1, PU-1:1 and PU-1:3. (b) FTIR spectra of Desmodur®L75 and Organosolv lignin (OL). The three different prepared PU formulations PU-3:1, PU-1:1 and PU-1:3 after air-drying at room temperature (c) and after oven-drying at 60 °C for 72 h (d).
References
- Tamantini, S.; Del Lungo, A.; Romagnoli, M.; Paletto, A.; Keller, M.; Bersier, J.; Zikeli, F. Basic Steps to Promote Biorefinery Value Chains in Forestry in Italy. Sustainability 2021, 13, 11731.
- Nitzsche, R.; Gröngröft, A.; Köchermann, J.; Meisel, K.; Etzold, H.; Verges, M.; Leschinsky, M.; Bachmann, J.; Saake, B.; Torkler, S.; et al. Platform and fine chemicals from woody biomass: Demonstration and assessment of a novel biorefinery. Biomass Convers. Biorefin. 2020, 11, 2369–2385.
- De Angelis, M.; Romagnoli, M.; Vek, V.; Poljanšek, I.; Oven, P.; Thaler, N.; Lesar, B.; Kržišnik, D.; Humar, M. Chemical composition and resistance of Italian stone pine (Pinus pinea L.) wood against fungal decay and wetting. Ind. Crop. Prod. 2018, 117, 187–196.
- Füchtner, S.; Brock-Nannestad, T.; Smeds, A.; Fredriksson, M.; Pilgård, A.; Thygesen, L.G. Hydrophobic and Hydrophilic Extractives in Norway Spruce and Kurile Larch and Their Role in Brown-Rot Degradation. Front. Plant Sci. 2020, 11, 1–20.
- Romagnoli, M.; Cavalli, D.; Pernarella, R.; Zanuttini, R.; Togni, M. Physical and mechanical characteristics of poor-quality wood after heat treatment. iForest-Biogeosci. For. 2015, 8, 884–891.
- Khan, A.; Huang, K.; Sarwar, M.G.; Cheng, K.; Li, Z.; Tuhin, M.O.; Rabnawaz, M. Self-healing and self-cleaning clear coating. J. Colloid Interface Sci. 2020, 577, 311–318.
- Jusic, J.; Tamantini, S.; Romagnoli, M.; Vinciguerra, V.; Di Mattia, E.; Zikeli, F.; Cavalera, M.; Scarascia Mugnozza, G. Improving sustainability in wood coating: Testing lignin and cellulose nanocrystals as additives to commercial acrylic wood coatings for bio-building. iForest-Biogeosci. For. 2021, 14, 499–507.
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092.
- Mattos, B.D.; Tardy, B.L.; Magalhães, W.L.E.; Rojas, O.J. Controlled release for crop and wood protection: Recent progress toward sustainable and safe nanostructured biocidal systems. J. Control. Release 2017, 262, 139–150.
- Papadopoulos, A.N.; Bikiaris, D.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and Chemical Modifications for Enhanced Key Wood Properties: A Review. Nanomaterials 2019, 9, 607.
- UNI EN 927-1:2013. Paints and Varnishes-Coating Materials and Coating Systems for Exterior Wood—Part 1: Classification and Selection; UNI: Milan, Italy, 2013.
- Singh, T.; Singh, A.P. A review on natural products as wood protectant. Wood Sci. Technol. 2011, 46, 851–870.
- Teacǎ, C.A.; Rosu, D.; Mustaţǎ, F.; Rusu, T.; Roşu, L.; Irina, R.; Varganici, C.-D. Natural bio-based products for wood coating and protection against degradation: A Review. BioResources 2019, 14, 4873–4901.
- Bulian, F.; Graystone, J.A. Chapter 3-raw materials for wood coatings (1)-film formers (binders, resins and polymers). In Wood Coatings; Bulian, F., Graystone, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 53–94.
- Ahvazi, B.; Wojciechowicz, O.; Ton-That, T.-M.; Hawari, J. Preparation of Lignopolyols from Wheat Straw Soda Lignin. J. Agric. Food Chem. 2011, 59, 10505–10516.
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242.
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and characterization of high boiling solvent lignin-based polyurethane film with lignin as the only hydroxyl group provider. RSC Adv. 2015, 5, 53949–53955.
- Wang, Y.-Y.; Cal, C.M.; Ragauskas, A.J. Recent advances in lignin-based polyurethanes. Tappi J. 2017, 16, 203–207.
- Stachak, P.; Łukaszewska, I.; Hebda, E.; Pielichowski, K. Recent Advances in Fabrication of Non-Isocyanate Polyurethane-Based Composite Materials. Materials 2021, 14, 3497.
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761.
- Wei, D.; Zeng, J.; Yong, Q. High-Performance Bio-Based Polyurethane Antismudge Coatings Using Castor Oil-Based Hyperbranched Polyol as Superior Cross-Linkers. ACS Appl. Polym. Mater. 2021, 3, 3612–3622.
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202.
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036.
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249.
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427.
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Unmodified kraft lignin isolated at room temperature from aqueous solution for preparation of highly flexible transparent polyurethane coatings. RSC Adv. 2018, 8, 40765–40777.
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154.
- Lang, J.M.; Shrestha, U.M.; Dadmun, M. The Effect of Plant Source on the Properties of Lignin-Based Polyurethanes. Front. Energy Res. 2018, 6, 1–12.
More
