Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ioannis Ilias and Version 2 by Lindsay Dong.
Metformin (MTF) occupies a major and fundamental position in the therapeutic management of type 2 diabetes mellitus (T2DM). Gender differences in some effects and actions of MTF have been reported. Women are usually prescribed lower MTF doses compared to men and report more gastrointestinal side effects. The incidence of cardiovascular events in women on MTF has been found to be lower to that of men on MTF.
- Metformin
- gender
- insulin resistance
1. Introduction: Metformin—Gender Medicine
Metformin (MTF) occupies a major and fundamental position in the therapeutic management of type 2 diabetes mellitus (T2DM) [1][2][3][1,2,3]. Sex pertains to “the different biological and physiological characteristics of males and females, such as reproductive organs, chromosomes or hormones”, whereas gender pertains to “the socially constructed characteristics of women and men—such as norms, roles and relationships of and between groups of women and men", quoting the relevant definitions from the Council of Europe (https://www.coe.int/en/web/gender-matters/sex-and-gender, accessed on 7 March 2022). Gender medicine is the medical discipline that integrates any effect of sex and/or gender on the overall level of health (prevention, diagnosis and treatment/management of diseases), taking into account biological as well as social sex differences [4]. Its aim is to improve health for any gender. Gender medicine may be a neglected dimension of medicine.
2. Pharmacokinetics, Pharmacodynamics and Metabolism of MTF
MTF is a weak base, and is very polar and extremely soluble in water [5]. It is absorbed from the small intestine, leading to a peak in concentration in one to two hours after oral intake. Its bioavailability is from 50% to 60% [6]. MTF is weakly bound to proteins. Its plasma half-life is estimated to be 1.5 to 5.0 h and it is practically unmetabolized after being distributed mainly in the liver, kidneys and intestine [7][8][9][7,8,9]. Excretion occurs via the kidneys, with a clearance of 933–1317 mL/min, involving glomerular filtration and tubular secretion [9]. The mechanisms of cellular action of MTF are still poorly understood. Various molecular responses are elicited by MTF and apparently some are influenced by sex hormones (Figure 1) [10][11][12][13][10,11,12,13]. The normoglycemic effect of MTF results mainly from a decrease in hepatic glucose production by inhibition of gluconeogenesis and by an action on glucose-6-phosphatase [2]. In addition to this action on the liver, which results mainly in a decrease in fasting blood sugar, MTF also potentiates the effect of insulin on muscle glucose uptake.
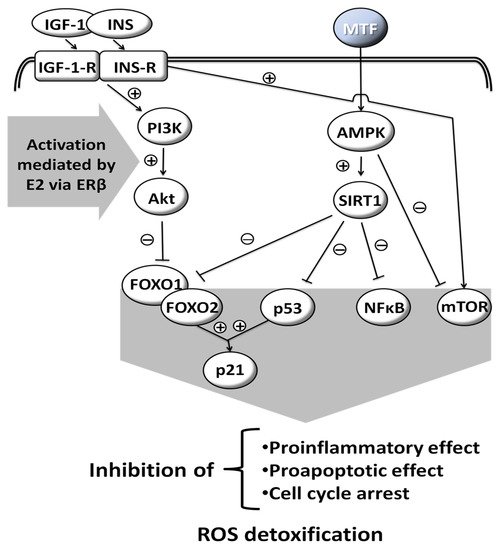

Figure 1. Molecular responses that are elicited by MTF; some are influenced by sex hormones. Insulin-like Growth Factor-1, INS: Insulin, IGF-1-R: Insulin-like Growth Factor-1-Receptor, INS-R: Insulin Receptor, PI3K: Phosphoinositide 3-kinase, Akt: Serine/threonine protein kinase B, AMPK: AMP-activated protein kinase, SIRT1: Sirtuin 1, FOXO1: Forkhead box protein O1, FOXO2: Forkhead box protein O2, p53: Tumor protein p53, NFκB: Nuclear factor kappa-light-chain-enhancer of activated B cells, mTOR: Mammalian target of rapamycin, p21: Cyclin-dependent kinase inhibitor 1, ROS: Reactive oxygen species, E2: Estradiol, ERβ: Estrogen receptor β, (+): activation, (−): inhibition, drawn with data from [10][11][12][13][10,11,12,13].
3. Gender-Specific Use of MTF
3.1. MTF in Women with Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disease (with variable prevalence worldwide, ranging from 6% to 26%) [14], which is characterized by anovulation, clinical or biochemical hyperandrogenemia and polycystic ovary morphology [15]. Hyperinsulinemia as a result of tissue insulin resistance, is central to PCOS [16]. Insulin resistance is observed in 45–65% of patients with PCOS and is associated with excessive phosphorylation of insulin receptors. Hyperinsulinemia, impaired glucose tolerance, dyslipidemia and hypertension affect 40–45% of patients with PCOS [17]. Hyperinsulinemia adversely affects the hypothalamic-pituitary-ovarian axis, resulting in altered endocrine control, menstrual irregularity and infertility [15].
Many interventional studies have demonstrated the positive effect of MTF on both the reproductive as well as metabolic aspects of the syndrome [15]. However, the mechanisms, by which MTF exerts its effects in treating PCOS, are only partially understood. The rationale of MTF use in PCOS is based on the fact that hyperinsulinemia is the basis of the syndrome and adversely affects ovarian function. Insulin boosts 17OH-progesterone activity causing ovarian stroma hypertrophy, follicular atresia and anovulation [15]. Therefore, MTF directly or indirectly improves steroidogenesis (this has been noted by in vitro studies of granuloma cell response to follicle stimulating hormone (FSH) and insulin growth factor 1 (IGF-1) [18]).
In 2013, the American Endocrine Society issued guidelines on the management of PCOS which included MTF as a treatment [19][24]. Specifically, MTF was recommended for women with PCOS and T2DM or insulin resistance, after failure of lifestyle change, diet and exercise, as a daily routine. It was not advised as a first line treatment of skin manifestations of PCOS (hair loss, acne), complications of the syndrome in pregnancy or for obesity [15]. MTF can also be given to women with menstrual disorders in which contraceptive treatment has failed or in women who wish to have children, as a second choice of treatment. There is no straightforward answer to whether all women with PCOS should undergo MTF treatment [20][25]. Proponents of MTF consider it a necessary drug for women with PCOS not only to prevent its long-term complications (in the context of insulin resistance) but also because MTF has been shown to improve all of the syndrome’s parameters. The first line treatment that includes diet and physical exercise is a time-consuming process that requires the compliance of women on a strict, long-term schedule, and relapse is very common.
Another element that comes to add to the beneficial action of MTF is in the treatment of adolescent girls with obesity and hyperandrogenemia. It seems that 50% of adolescent women with hyperandrogenemia have already developed resistance to progesterone-mediated gonadotropin-releasing hormone (GnRH) pulse suppression. The abnormal regulation of GnRH and luteinizing hormone (LH) secretion by the persistence of increased frequency of GnRH pulses is already present in adolescent girls with hyperandrogenemia before menarche [21][26]. Thus, the correction of androgen overproduction in PCOS is deemed to be necessary.
3.2. MTF in Women with Gestational Diabetes
The use of MTF in pregnancy is considered to be safe overall, with favorable effects on maternal weight gain, the incidence of preeclampsia, the dosage in concomitant insulin administration, and the rate of fetal macrosomia and neonatal hypoglycemia; it may increase the rate of small-for-gestational-age infants [22][23][24][25][26][27][28][34,35,36,37,38,39,40]. Apparently, MTF lowers proinflammatory cytokines (tumor necrosis factor alpha (TNF-α), interleukin [IL]-1-alpha and IL-1-beta and IL-6) in serum, placenta and omental tissues [29][41]. Interestingly, although in a very critical assessment of metanalyses regarding MTF, most were deemed to be of low quality, the exception being those in obstetric/gynecological settings [30][42]. There are caveats in the use of MTF in pregnancy: it was found—in vitro, in human embryonic stem cells—to decrease the differentiation of pancreatic beta cells [31][43], and in mice to decrease or arrest early embryonic development [32][44].
4. Sex/Gender Differences Using MTF
4.1. Prescribing/Administering MTF for Diabetes
There may be a difference in T2DM prevalence by sex/gender [33][45]; this difference depends on the definition of diabetes per se: men tend to have higher fasting plasma glucose more often, whereas women tend to have abnormalities in the oral glucose tolerance test (both modalities are used in the diagnosis of the disease) [34][46]. Although MTF is widely prescribed worldwide as a first-choice medication for T2DM [35][47], few studies have seen the light regarding use by gender. Although women are more concerned than men about their body image [36][48], and MTF may show a modest effect on weight loss [37][49], women are usually prescribed lower MTF doses compared to men (and they report more gastrointestinal side effects) [38][50].
4.2. MTF and Vitamin B12/Homocysteine
The long-term administration of MTF significantly lowers vitamin B12 levels [39][40][41][59,60,61]. Vitamin B12 deficiency with MTF is rarely symptomatic; it is linked to a reduction in the intestinal absorption of cobalamin and can be reversed by the discontinuation of MTF or with oral B12 supplementation. Men have lower vitamin B12 levels compared to women [42][62]. In a study of patients with T2DM (without a control group), higher doses of MTF and male sex were factors associated with lower levels of vitamin B12 [43][63]. Nevertheless, the effect of MTF on B12 by sex/gender, has not been assessed adequately; this is of interest given the sex/gender differences presented above. Additionally, MTF may conditionally elevate or reduce homocysteine levels, which is critical for people with obesity [44][45][64,65].
4.3. MTF and Cardiovascular Disease
MTF is considered to be associated with some degree of cardioprotection [46][47][66,67]; the latter is apparently the net result of its beneficial actions on endothelial and smooth muscle cells, blood lipids and systemic chronic inflammation [48][49][68,69]. In experimental models, MTF was beneficial with regards to myocardial reperfusion, fibrosis and inflammation in post-experimental myocardial ischemia [50][51][70,71]. In an older, small scale, study, MTF was noted to have a favorable effect on cardiac metabolism in women (increasing myocardial glucose uptake and lowering fat metabolism), in contrast to having an unfavorable one (with opposite effects) in men [52][72]. In a study of 167,254 (46% women) patients with T2DM who were already using MTF and started newer anti-diabetic medications, the incidence of cardiovascular events, after a median observation time of 4.5 years, in women was lower compared to that of men (14.7 versus 16.7 per 1000-person-year) [53][73]. Nevertheless, a systematic review of MTF’s overall actions has not been conclusive regarding micro- and macrovascular complications in patients with T2DM [54][74].
4.4. MTF and Andrology/Urology
In small (and—apparently—underpowered) studies, the effects of MTF solely on men have been observed. This medication has been reported to be of benefit in non-diabetic men with erectile dysfunction who had not responded to sildenafil [55][75]. The mechanisms are obscure: they may be direct, via endothelium-dependent vasodilatation or attenuation of sympathetic nerve activity, and indirect, via MTF’s effect on blood pressure [55][75]. Indirect proof of the low power of studies is that erectile dysfunction, low sex drive and low testosterone (total, free and bioavailable) have also been attributed to MTF use in men with T2DM [56][76]. Furthermore, the use of MTF for T2DM, in men with prostate cancer, has been associated with lower prostate-specific antigen levels and improved survival [55][57][58][59][75,77,78,79].
4.5. Musculoskeletal Effects of MTF
From in vitro studies, a role for MTF has been proposed in the stimulation of osteogenesis; in vivo studies are less conclusive [60][80]. Furthermore, MTF activates adenosine monophosphate-activated protein kinase (AMPK) signaling pathways. The activation of AMPK has been implicated in muscle repair [60][80]. Thus, it is not surprising that patients using MTF report less musculoskeletal pain vis-à-vis patients not on MTF [61][81]. The beneficial musculoskeletal effects of MTF were recently found to be more pronounced in women compared to men [61][81].
4.6. MTF and Experimentally-Induced Neurological Disease
An interesting dimorphism has been observed in mice regarding experimentally induced neuropathic pain and spinal cord microglial activation. MTF was shown to prevent and reverse neuropathic pain and spinal cord microglial activation only in male mice [62][82]. The researchers presume that the known activation of AMPK may be implicated, although no firm etiology for the sex difference in observations has been formulated. On the other hand, in another experimental study of brain injury in mice, MTF was beneficial for cognitive recovery in females but not males [63][83], pointing to a crucial relevant role for estradiol/testosterone [63][83].
4.7. MTF and Aging/Life Span (Experimental)
Dimorphic sex responses to MTF regarding life span have been described: chronic administration of MTF extended the lifespan of female mice and curtailed the lifespan of male mice [64][84]. Yet, more recent studies show that the positive effect of MTF on longevity is more prominent in male mice [65][85]. In the Mexican fruit fly the effect of MTF on longevity is dose-dependent, and is beneficial in higher doses for females and in lower doses for males [66][86].
4.8. MTF and Cancer
In older and newer studies, MTF in subjects with T2DM (in the older studies at low doses of 500 mg/day or less) was shown to be more beneficial vis-à-vis the incidence of colorectal cancer in women compared to men [67][68][87,88]. In men, MTF use may lower the risk of prostate cancer, but the effect—if any—is apparently slight and statistically non-significant [69][89]. In a Lithuanian cohort, the lowest risk for endometrial cancer was observed in diabetic women who used only MTF (with a standardized incidence ratio [SIR] of 1.69 and 95% confidence interval [CI] of 1.49 to 1.92) [70][90]. MTF was found to lower the markers of proliferation in endometrial cancer cells [71][72][91,92].
4.9. MTF and the Microbiome
Subtle differences have been reported in the gut microbiome between the male and female offspring of MTF-treated mice [73][101], as well as after MTF treatment in adult mice [74][102]. Before treatment with MTF and a high-fat diet (HFD), female mice had a preponderance of Lactobacillus species, whereas male mice had a preponderance of Proteobacteria species. After ten weeks of HFD and MTF, the bacterial species were different in males and females: a more pronounced increase in Bacteroides was noted in female mice compared to male ones [74][102].
4.10. MTF and COVID-19
Currently, there is a global effort to fight and win against the new severe acute respiratory syndrome coronvirus-2 (SARS-CoV-2) pandemic and its related coronavirus disease 2019 (COVID-19); proper management of T2DM is of even greater importance, since the presence of diabetes is associated with the most severe forms of COVID-19 and related mortality [75][76][108,109], and glycemic control is crucial [77][78][110,111]. In addition, a significant increase of cardiometabolic complications has been reported in many geographical areas, highlighting the need of a comprehensive and multidisciplinary approach to this terrible pandemic [79][80][112,113].
