Mesembryanthemum crystallinum L. is a nutritious edible facultative halophyte. They were cultivated with different percentages of artificial seawater (ASW). All plants were green and healthy. However, there were reductions in shoot and root productivity, and leaf growth. The concentrations of proline, ascorbic acid (ASC), and total phenolic compounds (TPC) increased as percentages of ASW increased. The salt-primed plants switched from C3 to crassulacean acid metabolism photosynthesis and accumulated the greatest amounts of proline, ASC, and TPC. In conclusion, higher salinities and salt priming enhance nutritional quality of M. crystallinum L. but compromises productivity.
- salinity stress
- salt priming
- phytochemicals
1. Introduction
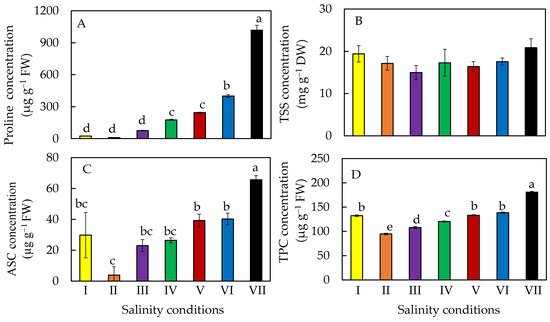
M. Crystallinum L. grown under 500 mM NaCl decreased leaf growth, biomass accumulation and leaf water content compared to those grown under lower concentration of NaCl. However, all plants were healthy with similar maximal efficiency of photosystem II (PS II) photochemistry. Plants grown under 500 mM NaCl switched from C3 photosynthesis to CAM photosynthesis in order to tolerate salinity stress. CAM is characterized by nocturnal CO2 uptake, which increased water-use efficiency when compared to C3 plants [27,[26][27]. 28]. Phytochemicals such as proline, total soluble sugar (TSS), ASC and total phenolic compounds (TPC) were significantly higher in plants grown under higher salinity compared to those grown under lower salinity [28][27].
2. Productivity

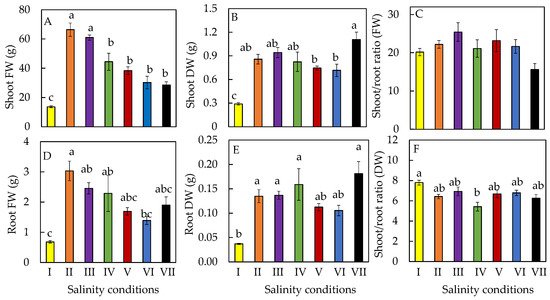
3. Leaf Growth
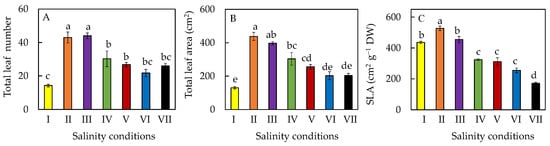
4. Accumulation of Phytochemicals
4. Researches and Findings
References
- Adams, P.; Nelson, D.E.; Yamada, S.; Chmara, W.; Jensen, R.G.; Bohnert, H.J.; Griffiths, H. Growth and development of Mesembryanthemum crystallinum. New Phytol. 1998, 138, 171–190.
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte common ice plants: A future solution to arable land salinization. Sustainability 2019, 11, 6076.
- Abd El-Gawad, A.M.; Shehata, H.S. Ecology and development of Mesembryanthemum crystallinum L. in the Deltaic Mediterranean coast of Egypt. Egy. J. Basic Appl. Sci. 2014, 1, 29–37.
- Ibtissem, B.; Abdelly, C.; Sfar, S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365.
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124.
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Q. Rev. Biol. 1986, 61, 313–337.
- Askari, H.; Edqvist, J.; Hajheidari, M.; Kafi, M.; Salekdeh, G.H. Effects of salinity levels on proteome of Suaeda aegyptiaca leaves. Proteomics 2006, 6, 2542–2554.
- Yu, J.; Chen, S.; Zhao, Q.; Wang, T.; Yang, C.; Diaz, C.; Sun, G.; Dai, S. Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J. Proteome Res. 2011, 10, 3852–3870.
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963.
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537.
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71.
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flower, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447.
- Stuchlík, M.; Žák, S. Vegetable lipids as components of functional foods. Biomed. Pap.-Palacky Univ. Olomouc 2002, 146, 3–10.
- Buhmann, A.; Papenbrock, J. An economic point of view of secondary compounds in halophytes. Func. Plant Biol. 2013, 40, 952–967.
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319.
- Cheeseman, J.M. The evolution of halophytes, glycophytes and crops, and its implications for food security under saline conditions. New Phytol. 2015, 206, 557–570.
- Abd Elgawad, H.; Zinta, G.; Hegab, M.M.; Pandey, R.; Asard, H.; Abuelsoud, W. High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front. Plant Sci. 2016, 7, 276.
- He, J.; You, X.; Qin, L. High salinity reduces plant growth and photosynthetic performance but enhances certain nutritional quality of C4 Halophyte Portulaca oleracea L. grown hydroponically under LED lighting. Front. Plant Sci. 2021, 12, 651341.
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83.
- Sivritepe, N.; Sivritepe, H.O.; Eris, A. The effects of NaCl priming on salt tolerance in melon seedlings grown under saline conditions. Sci. Hortic. 2003, 97, 229–237.
- Cayuela, E.; Perez-Alfocea, F.; Caro, M.; Bolarin, M.C. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol. Plant. 1996, 96, 231–236.
- Farhoudi, R.; Sharifzadeh, F.; Poustini, K.; Makkizadeh, M.T.; Kochakpor, M. The effects of NaCl priming on salt tolerance in canola (Brassica napus) seedlings grown under saline conditions. Seed Sci. Technol. 2007, 35, 754–759.
- Khan, H.A.; Ayub, C.M.; Pervez, M.A.; Bilal, R.M.; Shahid, M.A.; Ziaf, K. Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 28, 81–87.
- Farhoudi1, R.; Saeedipour, S.; Mohammadreza, D. The effect of NaCl seed priming on salt tolerance, antioxidant enzyme activity, proline and carbohydrate accumulation of Muskmelon (Cucumis melo L.) under saline condition. Afr. J. Agric. Res. 2011, 6, 1363–1370.
- He, J.; Qin, L. Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities. Acta. Hortic. 2020, 1296, 219–226.
- He, J.; Koh, D.J.Q.; Qin, L. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2021.
- Acosta-Motos, J.; Ortuño, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.; Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18.
- Franco, J.A.; Fernández, J.A.; Bañón, S.; González, A. Relationship between the effects of salinity on seedling leaf area and fruit yield of six muskmelons cultivars. J. Hortic. Sci. 1997, 32, 642–647.
- Rodríguez, P.; Torrecillas, A.; Morales, M.A.; Ortuño, M.F.; Sánchez-Blanco, M.J. Effects of NaCl salinity and water stress on growth and leaf water relations of Asteriscus maritimus plants. Environ. Exp. Bot. 2005, 53, 113–123.
- Muchate, N.S.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. NaCl induced salt adaptive changes and enhanced accumulation of 20-hydroxyecdysone in the in vitro shoot cultures of Spinacia oleracea (L.). Sci. Rep. 2019, 9, 12522.
- Haider, M.S.; Barnes, J.D.; Cushman, J.C.; Borland, A.M. A CAM- and starch-deficient mutant of the facultative CAM species Mesembryanthemum crystallinum reconciles sink demands by repartitioning carbon during acclimation to salinity. J. Exp. Bot. 2012, 63, 1985–1986.
- Winter, K.; Holtum, J.A.M. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441.
- He, J.; Qin, L. Productivity and photosynthetic characteristics of the facultative halophyte Mesembryanthemum crystallinum grown indoors with LED lighting under different salinities. Acta. Hortic. 2020, 1296, 219–226.
- He, J.; Koh, D.J.Q.; Qin, L. LED spectral quality and NaCl salinity interact to affect growth, photosynthesis and phytochemical production of Mesembryanthemum crystallinum. Funct. Plant Biol. 2021.
- Hsouna, A.B.; Ghneim-Herrera, T.; Romdhane, W.B.; Dabbous, A.; Saad, R.B.; Brini, F.; Abdelly, C.; Hamed, B.K. Early effects of salt stress on the physiological and oxidative status of the halophyte Lobularia maritima. Funct. Plant. Biol. 2020, 47, 912–924.
- Blum, A.; Munns, R.; Passioura, J.B.; Turner, N.C.; Sharp, R.E.; Boyer, J.S.; Nguyen, H.T.; Hsiao, T.C.; Verma, D.P.S.; Hong, Z. Genetically engineered plants resistant to soil drying and salt stress: How to interpret osmotic relations? Plant Physiol. 1996, 10, 1051–1053.
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374.
