1.1. Two Types of the Extracellular Matrix
Although the basic organisation of the
extracellular matrix (ECM
) structure is the same throughout, two basic types of the matrix are distinguished by their location and composition: the interstitial matrix, which forms a three-dimensional porous network surrounding the cells (especially connective tissues), and the pericellular matrix, which is more compact and forms a layer adjacent to the cells
[1][2][15,16] (see
Figure 1).
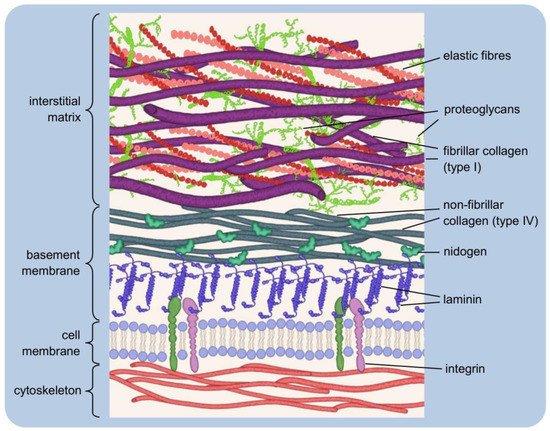
Figure 1. Simplified extracellular matrix structure: three-dimensional macromolecular network composed of various proteins and polysaccharides. The pericellular matrix forms a layer adjacent to the cells: integrins bind to polymerised laminin, which, in turn, is connected via nidogen to the type IV collagen. Interstitial matrix forms porous network of fibrillar collagens, elastic fibres, and proteoglycans.
The interstitial matrix can be equated with the “proper” matrix, as it forms the structural scaffolding for the cells. Its basic components are heterotypic fibrils, composed mainly of type I collagen with small amounts of type III and V collagens in variable proportions, both playing an important role in fibrillogenesis
[2][16]. The collagens of the interstitial matrix are mostly secreted by fibroblasts
[3][17]. Important components of this “amorphous three-dimensional gel” also include fibronectin and elastin, involved in the organisation of the structure
[4][5][18,19].
A typical example of the pericellular matrix is the basement membrane, a delicate and flexible nanostructure that separates the epithelium from the deeper layers of connective tissues. It ensheathes smooth, skeletal, and cardiac muscle fibres, Schwann cells, and adipocytes. The basement membrane forms a specific boundary of many organs in mature tissues, often surrounding their functional units
[2][6][7][8][16,20,21,22]. It is mainly composed of type IV collagen, laminins, nidogens and heparan sulfate proteoglycans (HSPGs): perlecan and agrin
[7][21]. The basement membrane contains so-called matricellular proteins that do not contribute to its physical stability or structural integrity, although they may be connected to building components. Instead, they have regulatory functions and interact with surface receptors, proteases, hormones or other biologically active molecules. They may be tissue-specific in terms of function and structure
[9][10][11][12][23,24,25,26]. Matricellular proteins include SPARC (secreted protein acidic and rich in cysteine, or osteonectin; characteristic of mineralising tissues, mainly bone), thrombospondin-1 (which is rich in platelet α-granules; when secreted, it causes, among other things, activation of TGF-β1, i.e., transforming growth factor-beta 1), and tenascin-C (the gene of this protein is expressed during embryonic life, while in adult tissues, tenascin-C is very poorly detectable, being present rather in the course of pathological processes
[13][14][15][27,28,29]. The tasks of the basement membrane include regulation of tissue development, function, and regeneration by controlling the cellular response. It is a storehouse of growth factors and modulates their activity and concentration. It serves to maintain the phenotype of the cells it surrounds
[16][30]. The interstitial matrix and the basement membrane are closely interconnected, ensuring the integrity of the tissue
[2][16].
The functional equivalent of the basement membrane described above is a type of pericellular matrix that surrounds chondrocytes in articular cartilage
[17][31]. It acts as a physical barrier that filters molecules entering and leaving the cells. Together with an adjacent thin layer of matrix, each chondrocyte forms a structural unit called a chondron
[18][32]. The morphology of chondrons varies. They can take a discoid/ellipsoid/rounded shape and a variable orientation, which depends on the position, i.e., the depth of location in the cartilage. In some cases, a chondron comprises more than one cell (up to four)
[19][33].
AIn this case, an essential component of the pericellular matrix is type VI collagen, although it generally constitutes a negligible percentage of the collagens of cartilage tissue
[20][34]. However, because of its specific presence in the chondrocyte environment of articular cartilage, it often serves as a marker of chondrons
[20][21][34,35]. A characteristic feature of articular cartilage is the small number of chondrocytes compared to the extensive extracellular (interstitial) matrix for which synthesis, organisation, and maintenance they are responsible
[20][34].
1.3. The Dynamic Structure of the Extracellular Matrix
The structure of the extracellular matrix undergoes continuous remodelling, during which changes in its composition and overall architecture occur. Cells embedded in the ECM are actively involved in its reorganisation. In addition to synthesising and secreting building components, they are also the source of enzymes that degrade these components. Remodelling processes are complex and must be tightly regulated to maintain environmental homeostasis
[5][147][148][149][19,160,161,162].
Protein-degrading enzymes belong to the class of hydrolases and are called proteases (proteinases). Depending on the mechanism of catalysis, they can be divided into several families, including serine proteases (serine residue in the enzyme active site), cysteine proteases (cysteine residue) or metalloproteases (they require the presence of a metal cation in the active centre). These enzymes can be secreted by the cell into its external environment or remain anchored in the cell membrane
[150][151][163,164].
The main group of enzymes involved in ECM degradation are the zinc-dependent matrix metalloproteinases (MMPs). More than 20 representatives of this group are known, capable of degrading different types of collagen, gelatin, elastin, laminin, fibronectin and many others
[152][153][154][165,166,167]. The sources of MMPs are mainly connective tissue cells (fibroblasts, osteoblasts), inflammatory cells (macrophages, neutrophils, mast cells), and endothelial cells
[152][155][165,168]. MMPs are secreted in the form of zymogens, inactive precursors that must undergo biochemical modifications to be activated
[5][152][155][19,165,168]. Through controlled degradation of ECM proteins, metalloproteinases facilitate cell migration and trigger the release of growth factors
[156][157][158][169,170,171]. They participate in tissue remodelling, an interesting example of which is postpartum uterine involution. In addition, they regulate angiogenesis (blood vessel formation), wound healing, embryonic development
, and so onetc.
[152][159][160][165,172,173]. In pathological states, their abnormal and/or increased activity contributes to the course of cardiovascular, cancer, autoimmune diseases
, and so onetc.
[152][161][162][163][165,174,175,176].
The proteolysis occurring in tissues relates not only to the extracellular matrix per se but also concerns the so-called ectodomain shedding, i.e., proteolytic cleavage of cell surface proteins. Modification, degradation, and changes in the activity of these proteins are one of the mechanisms of the cell’s response to changes in microenvironment conditions
[164][165][177,178]. Enzymes of the ADAM (a disintegrin and metalloproteases) family, also known as adamalysins, are mainly involved in this process. They have various functions, primarily engaged in intercellular interactions and signal transduction
[5][166][167][19,179,180]. The release of biologically active extracellular domains of multiple proteins (cytokines, adhesion molecules, growth factors) from the cell membrane can contribute, e.g., to inflammation (physiological and pathological), as occurs as a result of ADAM17 enzyme activity. The pro-inflammatory action of this sheddase consists of a modification of the cell surface and enrichment of its environment with active soluble molecules
[168][169][170][171][181,182,183,184]. The structure and function of ADAM group proteins are similar to the metalloproteinases found in snake venom, responsible for the typical effects of snakebites (haemorrhage, tissue necrosis)
[172][185].
1.4. The Extracellular Matrix as a Storehouse of Growth Factors
The ECM significantly influences the cell’s most important natural biological processes: growth, proliferation, and programmed death
[173][186]. In addition to mediating interactions and activating relevant mechanisms by contact with its building proteins, the ECM serves as a storehouse of growth factors (and proteases and protease inhibitors). These molecules can be released by proteolytic degradation of the matrix, and the degradation itself regulates the rate, site and intensity of such activation. The fact that growth factors are stored in the vicinity of cells favours increased specificity of their action
[5][174][175][176][19,187,188,189].
Growth factors are generally not freely dispersed in the extracellular space but bind, for example, to heparan sulphate proteoglycans. HSPGs then participate in the matrix storage function by preventing the movement and proteolysis of growth factors. They allow their controlled release when necessary. However, another role of HSPGs is also to bind to such molecules to activate them. Then, they act as a coreceptor in ligand–receptor interactions
[174][177][178][179][187,190,191,192]. The type of interaction of HSPGs with growth factors depends on the localisation of these proteoglycans. They may remain anchored to the cell membrane or form a structural component of the ECM
[180][193].
A well-studied group is the fibroblast growth factors (FGF), which include 22 proteins with key functions in cell development, morphogenesis, tissue repair processes, and angiogenesis. They are among the neurotrophic factors,
for i.e
xample., those that stimulate and regulate neurogenesis. Some are being investigated for involvement in the development of depression
[179][181][192,194]. FGF molecules are mainly bound by heparan sulfate and heparin chains
[182][183][195,196]. Proteolytic release of FGF allows subsequent binding of FGF ligands to receptors on the cell surface. This stimulates cell signalling
[96][2].