Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Jae-Ku Oem.
Avian influenza viruses (AIVs) are eight segmented, single-stranded negative-sense RNA viruses. Based on viral surface proteins, hemagglutinin (HA) and neuraminidase (NA), influenza viruses are classified into 18 HA and 11 NA subtypes. H1-16 and N1-9 have been detected in avian species, but H17-18 and N10-11 have been discovered only in bats. In particular, H5 and H7 are important subtypes because they have the potential to mutate into highly pathogenic avian influenza viruses (HPAIVs), causing severe clinical signs in poultry. According to previous research, the H7 low pathogenic avian influenza virus (LPAIV) is a precursor to the H7 HPAIV.
- avian influenza virus
- H7N7
- H7N9
- wild bird
1. Introduction
H7 LPAIVs have been detected in poultry farms worldwide, and wild birds are regarded as the origin of H7 LPAIVs [16][1]. In wild birds, all nine neuraminidase (N1–N9) are found in H7 AIVs, and H7N7 viruses are the subtypes reported in the largest number of countries geographically [17][2]. In South Korea, two LPAIVs, H7N3 and H7N8, were detected in domestic ducks in 2007 [18][3]. Since then, through active national surveillance, H7 LPAIVs have been isolated continuously from poultry farms and wild birds, and H7N7 viruses are the most common subtypes in wild birds [19,20][4][5]. Viruses isolated from poultry are closely related to wild bird isolates [19][4]. Moreover, some H7 LPAIVs isolated from wild birds showed pathogenicity in chickens in laboratory experiments.
AIVs can cause cross-species transmission from birds to mammals [21][6]. Avian-like H7N7 viruses have been detected in equines and pinnipeds [22][7]. In addition, there are many cases of H7 AIV infection in humans. The first case of H7N7 LPAIV directly transmitted from avian to human was reported in 1996 [23][8]. The infected woman had contact with a duck; she suffered from conjunctivitis and recovered naturally. In 2003, 89 cases of H7N7 HPAIV human infections occurred in the Netherlands [24][9]. One of the 89 patients died of acute respiratory distress syndrome, and three were confirmed to have contracted the virus through transmission between humans. A recent human infection of H7N7 AIVs was observed in three poultry workers in Italy in 2013, and they showed only conjunctivitis but no respiratory syndromes [25][10].
In 2013, the first case of human infection with H7N9 AIVs was reported in Shanghai, and since then, the H7N9 virus has shown five epidemic patterns in China [26,27][11][12]. The H7N9 viruses that occurred in 2013 were low pathogenic viruses but gradually mutated, resulting in high pathogenic strains in 2017 [6,28][13][14]. By 2019, 1568 cases of H7N9 human infections were confirmed worldwide, of which 616 deaths occurred [29][15]. However, after the introduction of H7N9 vaccine in poultry in 2017 in China, human infection with H7N9 virus has decreased dramatically [30,31][16][17]. Since September 2019, no human infection has been reported according to the FAO H7N9 situation update. Moreover, human infections related to other subtypes of H7 LPAIVs, such as H7N2 and H7N3, have often been reported since the 2000s [32][18]. Severe human infection with H7N4 originating from backyard poultry was reported in Jiangsu in 2018 [33][19].
In 2021, two AIVs, H7N7 and H7N9, were isolated from wild birds in South Korea. The molecular and phylogenetic characterizations of the two H7 Korean AIVs were analyzed in this study. In addition, pathogenicity in mammals was evaluated using a murine model.
2. Molecular Characterization of H7 AIV Isolates
The whole genomes of 20X-20 and 34X-2 viruses were obtained and deposited in GenBank with the accession numbers MZ803114-MZ803121 and MZ803125-MZ803132, respectively. The obtained full gene sequences of 20X-20 and 34X-2 were compared with four selected reference H7 viruses: A/chicken/Jiangsu/1/2018 (H7N4; chicken/1), A/Jiangsu/1/2018 (H7N4; Jiangsu/1), A/Anhui/1/2013 (H7N9; Anhui/1), and A/Italy/3/2013 (H7N7; Italy/3) (Table 1) (14, 15, 19). The Jiangsu/1, Anhui/1, and Italy/3 viruses originate from the H7 AIV and cause human infections. The chicken/1 virus was isolated from chickens in the backyard of humans infected with the Jiangsu/1 virus. The amino acid sequences of the HA cleavage sites of 20X-20 and 34X-2 were ELPKGR/GLF, indicating they are LPAIVs. The amino acids at positions 186, 190, 225, 226, 227, and 228 of the HA gene (numbering based on H3) are related to host receptor binding efficiency [43,44,45][20][21][22]. Except for 186V and 226L in Anhui/1, all H7 viruses had 186G, 190E, 225G, 226Q, 227S, and 228G, indicating that they prefer avian receptors over human receptors. NA stalk deletions (69–73) were not detected in any of the H7 viruses except for the Anhui/1 virus. However, drug resistance-associated mutations were identified at the 117 position in the NA genes of 20X-20 and 34X-2.
Table 1.
Molecular characterization of H7 avian influenza virus (AIV) isolates.
| Viral Protein | Amino Acid Residue | Virus Strains | Comments | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20X-20 (2021) | 34X-2 (2021) |
Chicken/1 (2018) |
Jiangsu/1 (2018) |
Anhui/1 (2013) |
Italy/3 (2013) |
||||||||
| HA | Cleavage site | ELPKGR/GLF | ELPKGR/GLF | ELPKGR/GLF | ELPKGR/GLF | ELPKGR/GLF | ETPKRRERR/GLF | LPAIV -monobasic |
[49] | [23] | |||
| A/red-crowned crane/South Korea/H1026/2017(H7N7) | 96.6% | G186V | G | G | G | G | V | G | Increased α2-6 binding | [45] | [22] | ||
| PB1 | A/red-crowned crane/South Korea/H1026/2017(H7N7) | 95% | A/wild_duck/South_Korea/KNU18-104/2018(H7N7) | 93.6% | E190D | E | E | E | E | E | |||
| PA | A/wild_duck/South_Korea/KNU18-106/2018(H7N7) | 99.1% | A/wild_bird/Eastern_China/1758/2017(H5N3) | 98.6% | E | [ | 43] | [20] | |||||
| Q/G225D | G | G | G | G | G | ||||||||
| HA | G | [ | A/wild_duck/South_Korea/KNU18-106/2018(H7N7) | 43 | ] | [ | 20] | ||||||
| 97.8% | A/wild_duck/South_Korea/KNU18-106/2018(H7N7) | 97.3% | Q226L | Q | Q | Q | Q | ||||||
| NP | A/common teal/Shanghai/NH110923/2019(H1N1) | 98.8% | A/duck/Mongolia/926/2019(H5N3) | L | Q | [ | 43] | [ | 99.3%20] | ||||
| S227N | S | S | S | S | S | S | |||||||
| NA | [ | 44 | ] | [ | 21 | ] | |||||||
| A/Anas platyrhynchos/South Korea/JB31-96/2019(H11N9) | G228S | G | G | G | G | G | G | [43] | [20] | ||||
| 98.2% | A/mallard/Korea/A15/2016(H7N7) | 97.4% | PB2 | L89V | V | V | V | V | V | V | Increased polymerase activity in mammalian cell lines and mice | [46] | [24] |
| I147T | I | I | I | I | I | T | [50] | [25] | |||||
| I292V | I | I | I | I | V | I | [51] | [26] | |||||
| G309D | D | D | D | D | D | D | [46] | [24] | |||||
| T339K | K | K | K | K | K | K | [46] | [24] | |||||
| K389R | R | R | R | R | K | R | [52] | [27] | |||||
| E627K | E | E | E | K | K | E | Increased virulence in mice | [53] | [28] | ||||
| D701N | D | D | D | D | D | D | [54,55] | [29][30] | |||||
| V598T/I | T | T | T | T | V | T | Increased polymerase activity in mammalian cell line | [52] | [27] | ||||
| PB1 | C38Y | Y | Y | Y | Y | Y | Y | Increased polymerase activity in mammalian cell line and pathogenicity in chicken | [56] | [31] | |||
| D622G | G | G | G | G | |||||||||
| MP | A/duck/Mongolia/916/2018(H3N8) | 99.7% | A/northern pintail/Alaska/362/2013(H3N8) | 99.4% | |||||||||
| NS | A/duck/Bangladesh/37509/2019(H8N4) | 99.6% | A/duck/Bangladesh/37509/2019(H8N4) | 99.6% | G | G | Increased polymerase activity and virulence in mice | [57] | [32] | ||||
| PB1-F2 | N66S | S | S | N | N | N | N | Increased virulence in mice | [48] | [33] | |||
| PA | S37A | A | A | A | A | S | A | Increased polymerase activity in mammalian cell line | [58] | [34] | |||
| K142R | R | K | K | K | K | K | [55] | [30] | |||||
| N383D | D | D | D | D | D | D | Increased pathogenicity in ducks | [59] | [35] | ||||
| N409S | S | S | S | S | N | S | Increased polymerase activity in mammalian cell line | [58] | [34] | ||||
| NP | I41V | I | I | I | I | I | I | Increased polymerase activity in mammalian cell line | [60] | [36] | |||
| M105V | M | V | M | M | V | V | Increased virulence in chicken | [61] | [37] | ||||
| A184K | K | K | K | K | K | K | [62] | [38] | |||||
| F253I | I | I | I | I | I | I | Increased virulence in mice | [63] | [39] | ||||
| V286A | A | A | A | A | A | A | [64] | [40] | |||||
| M437T | T | T | T | T | T | T | |||||||
| NA | 69–73 deletion (QISNT) |
No | No | No | No | Yes | No | Increased virulence in mice | [65] | [41] | |||
| I117T | T | T | I | I | T | T | Increased resistance to antiviral drugs (oseltamivir and zanamivir) | [66] | [42] | ||||
| M1 | N30D | D | D | D | D | D | D | Increased virulence in mice | [50] | [25] | |||
| I43M | M | M | M | M | M | M | [67] | [43] | |||||
| T215A | A | A | A | A | A | A | [50] | [25] | |||||
| M2 | L26F | L | L | L | L | L | L | Increased resistance to antiviral drugs (amantadine and rimantadine) | [68] | [44] | |||
| S31N | S | S | S | S | N | S | [68] | [44] | |||||
| NS1 | P42S | S | S | S | S | S | S | Increased virulence in mice | [69] | [45] | |||
| D92E | D | D | D | D | D | D | Increased virulence in mice | [70] | [46] | ||||
| L103F | F | F | F | F | L | F | Increased replication and virulence in mice | [71] | [47] | ||||
| C138F | F | F | F | F | F | F | Increased replication in mammalian cell line | [72] | [48] | ||||
The amino acid mutations of the two H7 Korean AIVs were mostly similar, except for K142R in the PA gene of 20X-20 and M105V in the NP gene of 34X-2. The PA gene of the two H7 Korean AIVs was clustered in the same subgroup in the phylogenetic tree but showed a different amino acid mutation at the 142 position. The amino acid substitutions of E627K in the PB2 gene, known to increase virulence in mice and human adaptation markers, were observed in Jiangsu/1 but not in the two H7 Korean AIVs [46,47][24][49]. Additionally, the amino acid substitutions of I292V of the PB2 gene, which increase the polymerase activity in both and avian cells were not identified in the two H7 Korean AIVs. The amino acid substitutions of N66S in the PB1-F2 gene, which increased virulence in mice, were observed in the two H7 Korean AIVs compared to the chicken/1, which most recently caused human infections [48][33]. The other mutations related to the virulence of the virus and drug resistance are shown in Table 1.
3. Phylogenetic Analysis of H7 AIVs Isolates
An ML tree analysis of the HA gene showed that the HA genes of 20X-20 and 34X-2 belonged to the Eurasian lineage (Figure 1). The HA genes of 20X-20 and 34X-2 showed 98.5% nucleotide identities with each other and were most closely related to A/wild duck/South Korea/KNU18-106/2018(H7N7), with identities of 97.8% and 98.5%, respectively (Table 2). Notably, the two H7 Korean AIVs showed high nucleotide similarity to the A/Jiangsu/1/2018(H7N4) virus (97.5% and 97%, respectively), which are human influenza viruses originating from AIVs. However, the HA genes of 20X-20 and 34X-2 viruses showed low nucleotide similarity to the HA gene of A/Italy/3/2013(H7N7) (90.2% each) and A/Anhui/1/2013(H7N9) (88.6% and 88.9%, respectively), which cause human infections.
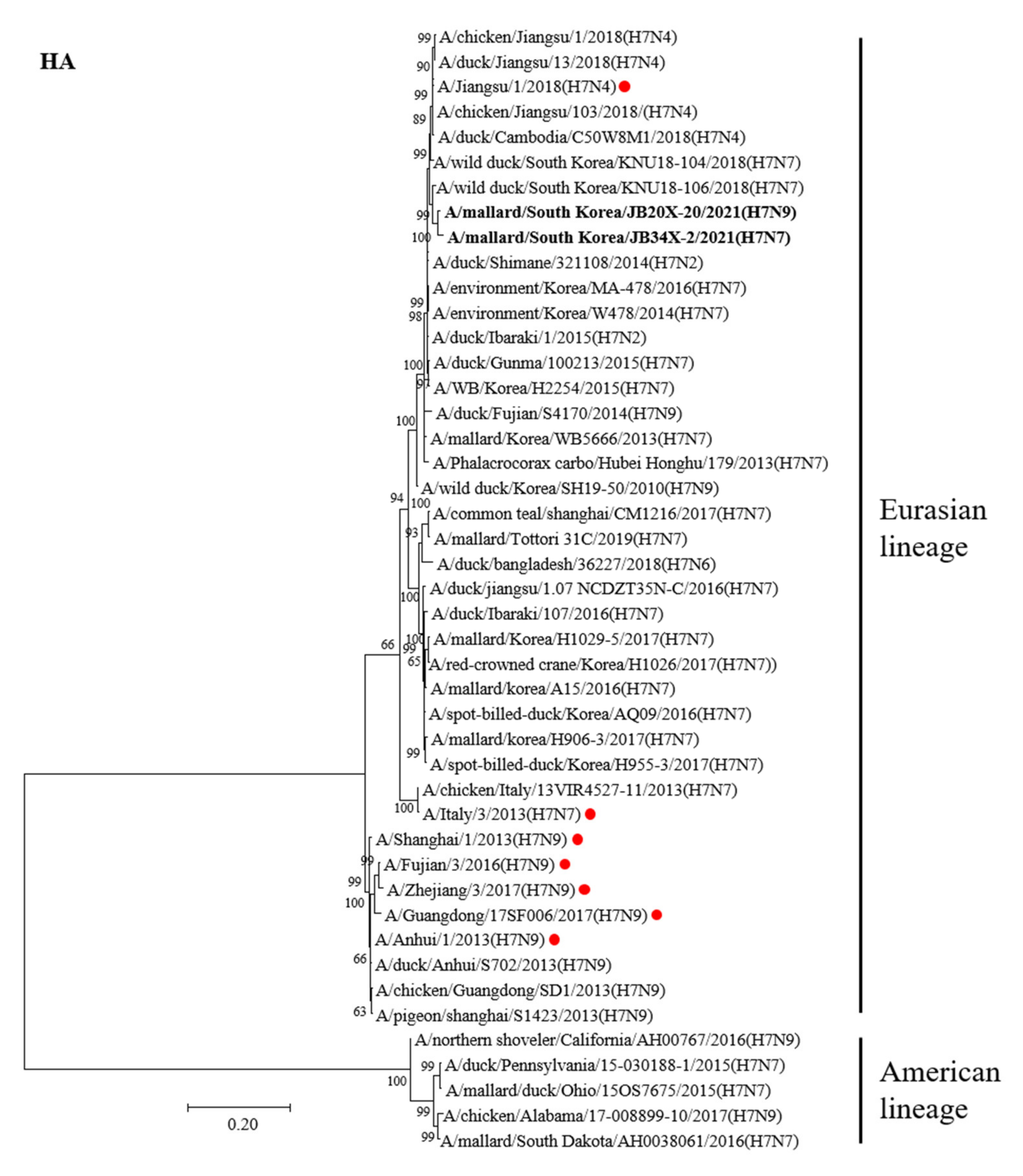

Figure 1. Phylogenetic tree of the HA gene of the H7 avian influenza virus (AIV) nucleotides. The tree was analyzed using the maximum-likelihood (ML) method with 1000 bootstrap replication and only bootstrap values more than 50% are shown. The two H7 Korea AIVs isolated in this study are shown in bold. The human influenza viruses are indicated by red circles.
Table 2. Sequence identities of the A/mallard/South Korea/JB20X-20/2021 (20X-20) and A/mallard/South Korea/JB34X-2/2021 (34X-2) genome.
| Gene | 20X-20 | Genetic Identity | 34X-2 | Genetic Identity |
|---|---|---|---|---|
| PB2 | A/wild_duck/South_Korea/KNU18-106/2018(H7N7) | 98.3% |
To estimate the times of origins of the HA gene of the two H7 Korean AIVs, wethe MCC tree was reconstructed the MCC tree. As the HA gene of the two H7 Korean AIVs belonged to the Asian lineage, we it was reconstructed the MCC tree using the H7 gene of LPAIVs isolated between 2016 and 2021 in Asia. In the MCC tree analysis, HA genes formed two distinct genetic subgroups (G1 and G2) (Figure 2). The H7 LPAIV in the G1 subgroup was identified as the one circulating in South Korea in 2017, and the H7 LPAIVs in the G2 subgroup were closely related to the H7 LPAIV isolated in China between 2016 and 2017. The HA genes of 20X-20 and 34X-2 belonged to the G1 subgroup.
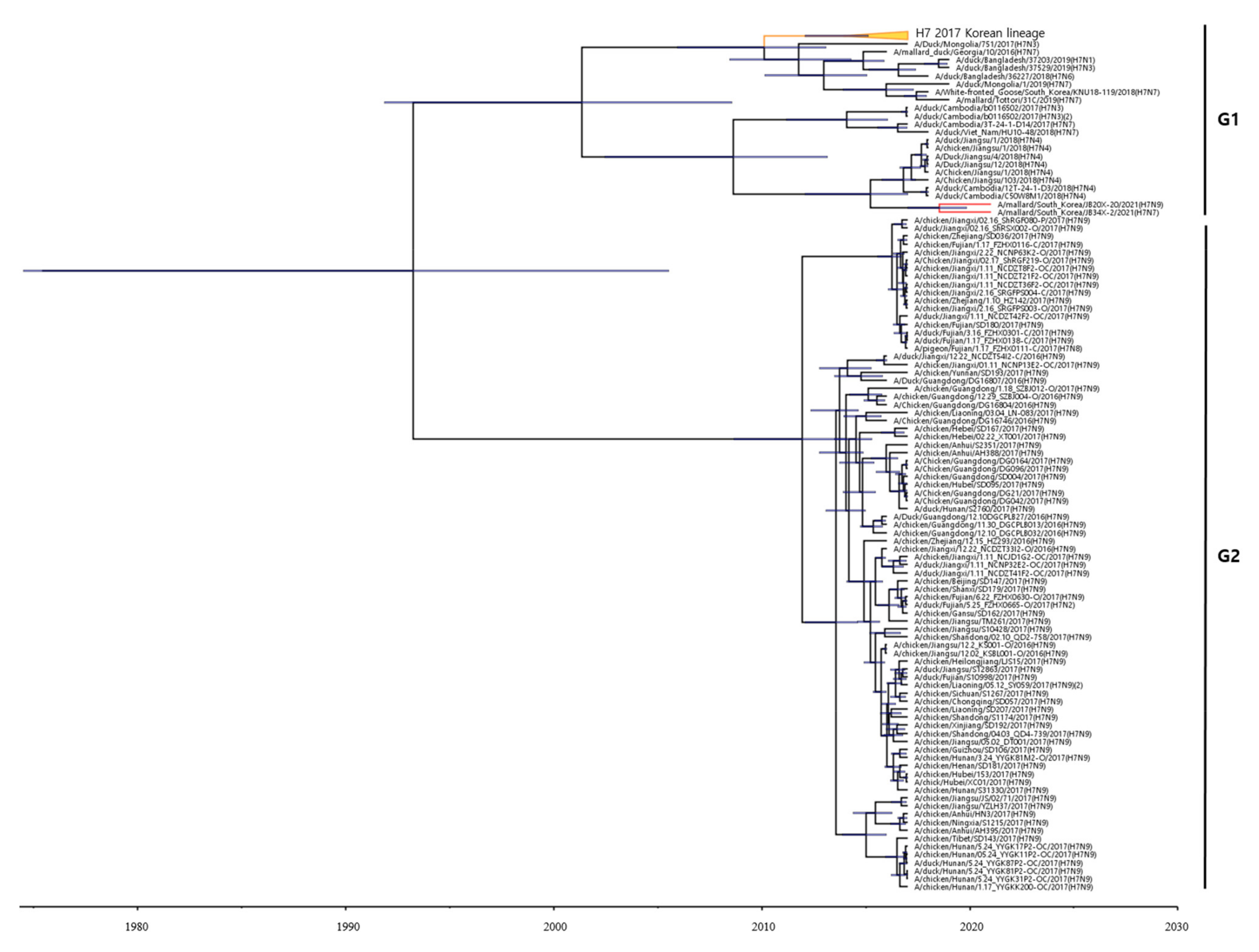

Figure 2. Maximum clade credibility (MCC) trees of the HA gene of H7 LPAIVs isolated between 2016 and 2021 in Asia. The MCC tree were constructed using the uncorrelated lognormal distribution relaxed clock method in BEAST v1.10.4. The ESS values were greater than 200. Posterior probabilities >0.8 are provided in the tree. The horizontal axis indicates the time scale, and the unit is 10 years. The two H7 Korean AIVs are shown in red. The H7 LPAIVs circulating in South Korea in 2017 are shown in orange.
The results of the phylogenetic tree of the NA gene showed that the NA gene of the two H7 Korean AIVs belonged to the Eurasian lineage (Supplementary Materials Figure S1). The NA genes of 20X-20 and 34X-2 showed the highest nucleotide identities with the NA gene of A/Anas platyrhynchos/South Korea/JB31/96/2019(H11N9) (98.2%) and A/mallard/Korea/A15/2016(H7N7) (97.4%), respectively. However, the NA gene of the two H7 Korean AIVs clustered distinct subgroups with human influenza viruses.
Most of the internal genes (PB2, PB1, PA, NP, M, and NS) of the two H7 Korean AIVs belonged to the Eurasian lineage, except for the M gene of 34X-2 (Supplementary Materials Figure S2). The PB2, PB1, PA, NP, M, and NS genes of 20X-20 and 34X-2 shared 95.1%. 95%, 96.7%, 94.1%, 93.9%, and 99.5% nucleotide identities, respectively. The NP and M genes, which showed less than 95% nucleotide similarity, were clustered into different subgroups. The PA gene of 20X-20 was clustered into the same subgroup as the PA gene of A/Mandarin duck/Korea/WB246/2016(H5N6), which is an internal gene of HPAIV.
References
- Naguib, M.M.; Verhagen, J.H.; Mostafa, A.; Wille, M.; Li, R.; Graaf, A.; Järhult, J.D.; Ellström, P.; Zohari, S.; Lundkvist, Å.; et al. Global patterns of avian influenza A (H7): Virus evolution and zoonotic threats. FEMS Microbiol. Rev. 2019, 43, 608–621.
- Abdelwhab, E.M.; Veits, J.; Mettenleiter, T.C. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiol. Infect. 2014, 142, 896–920.
- Kim, M.-C.; Jeong, O.-M.; Kang, H.-M.; Paek, M.-R.; Kwon, J.-S.; Song, C.-S.; Kwon, Y.-K.; Lee, J.-G.; Kwon, J.-H.; Lee, Y.-J. Pathogenicity and transmission studies of H7N7 avian influenza virus isolated from feces of magpie origin in chickens and magpie. Vet. Microbiol. 2010, 141, 268–274.
- Kim, H.-R.; Park, C.-K.; Lee, Y.-J.; Oem, J.-K.; Kang, H.-M.; Choi, J.-G.; Lee, O.-S.; Bae, Y.-C. Low pathogenic H7 subtype avian influenza viruses isolated from domestic ducks in South Korea and the close association with isolates of wild birds. J. Gen. Virol. 2012, 93, 1278–1287.
- Lee, E.-K.; Lee, Y.-N.; Kye, S.-J.; Lewis, N.S.; Brown, I.H.; Sagong, M.; Heo, G.-B.; Kang, Y.-M.; Cho, H.-K.; Kang, H.-M.; et al. Characterization of a novel reassortant H5N6 highly pathogenic avian influenza virus clade 2.3.4.4 in Korea, 2017. Emerg. Microbes Infect. 2018, 7, 103.
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R.A.M. Avian influenza virus transmission to mammals. In Influenza Pathogenesis and Control; Current Topics in Microbiology and Immunology 385; Springer: Berlin, Germany, 2014; Volume I, pp. 137–155.
- Reperant, L.; Rimmelzwaan, G.; Kuiken, T. Avian influenza viruses in mammals. Rev. Sci. Tech. L’oie 2009, 28, 137–159.
- Kurtz, J.; Manvell, R.J.; Banks, J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet 1996, 348, 901–902.
- Fouchier, R.A.M.; Schneeberger, P.M.; Rozendaal, F.W.; Broekman, J.M.; Kemink, S.A.G.; Munster, V.; Kuiken, T.; Rimmelzwaan, G.F.; Schutten, M.; van Doornum, G.J.J.; et al. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 1356–1361.
- Puzelli, S.; Rossini, G.; Facchini, M.; Vaccari, G.; Di Trani, L.; Di Martino, A.; Gaibani, P.; Vocale, C.; Cattoli, G.; Bennett, M.; et al. Human infection with highly pathogenic A (H7N7) avian influenza virus, Italy, 2013. Emerg. Infect. Dis. 2014, 20, 1741–1745.
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897.
- Wu, X.; Xiao, L.; Li, L. Research progress on human infection with avian influenza H7N9. Front. Med. 2020, 14, 8–20.
- Shi, J.; Deng, G.; Kong, H.; Gu, C.; Ma, S.; Yin, X.; Zeng, X.; Cui, P.; Chen, Y.; Yang, H.; et al. H7N9 virulent mutants detected in chickens in China pose an increased threat to humans. Cell Res. 2017, 27, 1409–1421.
- Shi, J.; Deng, G.; Ma, S.; Zeng, X.; Yin, X.; Li, M.; Zhang, B.; Cui, P.; Chen, Y.; Yang, H.; et al. Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host Microbe 2018, 24, 558–568.e7.
- Philippon, D.A.M.; Wu, P.; Cowling, B.J.; Lau, E.H.Y. Avian influenza human infections at the human-animal interface. J. Infect. Dis. 2020, 222, 528–537.
- Yin, X.; Deng, G.; Zeng, X.; Cui, P.; Hou, Y.; Liu, Y.; Fang, J.; Pan, S.; Wang, D.; Chen, X.; et al. Genetic and biological properties of H7N9 avian influenza viruses detected after application of the H7N9 poultry vaccine in China. PLoS Pathog. 2021, 17, e1009561.
- Zeng, X.; Tian, G.; Shi, J.; Deng, G.; Li, C.; Chen, H. Vaccination of poultry successfully eliminated human infection with H7N9 virus in China. Sci. China Life Sci. 2018, 61, 1465–1473.
- Belser, J.A.; Bridges, C.B.; Katz, J.M.; Tumpey, T.M. Past, present, and possible future human infection with influenza virus a subtype H7. Emerg. Infect. Dis. 2009, 15, 859–865.
- Huo, X.; Cui, L.-B.; Chen, C.; Wang, D.; Qi, X.; Zhou, M.-H.; Guo, X.; Wang, F.; Liu, W.J.; Kong, W.; et al. Severe human infection with a novel avian-origin influenza A(H7N4) virus. Sci. Bull. 2018, 63, 1043–1050.
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza a virus species specificity. Nat. Rev. Microbiol. 2019, 17, 67–81.
- Chen, L.M.; Blixt, O.; Stevens, J.; Lipatov, A.S.; Davis, C.T.; Collins, B.E.; Cox, N.J.; Paulson, J.C.; Donis, R.O. In vitro evolution of H5N1 avian influenza virus toward human-type receptor specificity. Virology 2012, 422, 105–113.
- Shi, Y.; Zhang, W.; Wang, F.; Qi, J.; Wu, Y.; Song, H.; Gao, F.; Bi, Y.; Zhang, Y.; Fan, Z.; et al. Structures and receptor binding of hemagglutinins from human-infecting H7N9 influenza viruses. Science 2013, 342, 243–247.
- Senne, D.A.; Panigrahy, B.; Kawaoka, Y.; Pearson, J.E.; Süss, J.; Lipkind, M.; Kida, H.; Webster, R.G. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: Amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 1996, 40, 425–437.
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129.
- Fan, S.; Hatta, M.; Kim, J.H.; Halfmann, P.; Imai, M.; Macken, C.A.; Le, M.Q.; Nguyen, T.; Neumann, G.; Kawaoka, Y. Novel residues in avian influenza virus PB2 protein affect virulence in mammalian hosts. Nat. Commun. 2014, 5, 5021.
- Kong, H.; Ma, S.; Wang, J.; Gu, C.; Wang, Z.; Shi, J.; Deng, G.; Guan, Y.; Chen, H. Identification of key amino acids in the PB2 and M1 proteins of H7N9 influenza virus that affect its transmission in guinea pigs. J. Virol. 2019, 94, e01180-19.
- Hu, M.; Yuan, S.; Zhang, K.; Singh, K.; Ma, Q.; Zhou, J.; Chu, H.; Zheng, B.-J. PB2 substitutions V598T/I increase the virulence of H7N9 influenza A virus in mammals. Virology 2017, 501, 92–101.
- Subbarao, E.K.; London, W.; Murphy, B.R. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993, 67, 1761–1764.
- Gao, Y.; Zhang, Y.; Shinya, K.; Deng, G.; Jiang, Y.; Li, Z.; Guan, Y.; Tian, G.; Li, Y.; Shi, J.; et al. Identification of amino acids in HA and PB2 critical for the transmission of H5N1 avian influenza viruses in a mammalian host. PLoS Pathog. 2009, 5, e1000709.
- Liang, L.; Jiang, L.; Li, J.; Zhao, Q.; Wang, J.; He, X.; Huang, S.; Wang, Q.; Zhao, Y.; Wang, G.; et al. Low polymerase activity attributed to pa drives the acquisition of the PB2 E627K mutation of H7N9 avian influenza virus in mammals. Mbio 2019, 10, e01162-19.
- Suzuki, Y.; Uchida, Y.; Tanikawa, T.; Maeda, N.; Takemae, N.; Saito, T. Amino acid substitutions in PB1 of avian influenza viruses influence pathogenicity and transmissibility in chickens. J. Virol. 2014, 88, 11130–11139.
- Feng, X.; Wang, Z.; Shi, J.; Deng, G.; Kong, H.; Tao, S.; Li, C.; Liu, L.; Guan, Y.; Chen, H. Glycine at position 622 in PB1 contributes to the virulence of H5N1 avian influenza virus in mice. J. Virol. 2016, 90, 1872–1879.
- Conenello, G.M.; Zamarin, D.; Perrone, L.A.; Tumpey, T.; Palese, P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza a viruses contributes to increased virulence. PLoS Pathog. 2007, 3, e141.
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza a viruses. J. Virol. 2014, 88, 3127–3134.
- Song, J.; Feng, H.; Xu, J.; Zhao, D.; Shi, J.; Li, Y.; Deng, G.; Jiang, Y.; Li, X.; Zhu, P.; et al. The PA protein directly contributes to the virulence of H5N1 avian influenza viruses in domestic ducks. J. Virol. 2011, 85, 2180–2188.
- Zhu, W.; Zou, X.; Zhou, J.; Tang, J.; Shu, Y. Residues 41V and/or 210D in the NP protein enhance polymerase activities and potential replication of novel influenza (H7N9) viruses at low temperature. Virol. J. 2015, 12, 71.
- Tada, T.; Suzuki, K.; Sakurai, Y.; Kubo, M.; Okada, H.; Itoh, T.; Tsukamoto, K. NP body domain and PB2 contribute to increased virulence of H5N1 highly pathogenic avian influenza viruses in chickens. J. Virol. 2010, 85, 1834–1846.
- Wasilenko, J.L.; Sarmento, L.; Pantin-Jackwood, M.J. A single substitution in amino acid 184 of the NP protein alters the replication and pathogenicity of H5N1 avian influenza viruses in chickens. Arch. Virol. 2009, 154, 969–979.
- Li, J.; Zheng, W.; Hou, L.; Chen, C.; Fan, W.; Qu, H.; Jiang, J.; Liu, J.; Gao, G.F.; Zhou, J.; et al. Differential nucleocytoplasmic shuttling of the nucleoprotein of influenza a viruses and association with host tropism. Cell. Microbiol. 2016, 19, e12692.
- Ma, S.; Zhang, B.; Shi, J.; Yin, X.; Wang, G.; Cui, P.; Liu, L.; Deng, G.; Jiang, Y.; Li, C.; et al. Amino acid mutations A286V and T437M in the nucleoprotein attenuate H7N9 viruses in mice. J. Virol. 2020, 94, e01530-19.
- Bi, Y.; Xiao, H.; Chen, Q.; Wu, Y.; Fu, L.; Quan, C.; Wong, G.; Liu, J.; Haywood, J.; Liu, Y.; et al. Changes in the length of the neuraminidase stalk region impact H7N9 virulence in mice. J. Virol. 2016, 90, 2142–2149.
- Kode, S.S.; Pawar, S.D.; Tare, D.S.; Keng, S.S.; Hurt, A.C.; Mullick, J. A novel I117T substitution in neuraminidase of highly pathogenic avian influenza H5N1 virus conferring reduced susceptibility to oseltamivir and zanamivir. Vet. Microbiol. 2019, 235, 21–24.
- Nao, N.; Kajihara, M.; Manzoor, R.; Maruyama, J.; Yoshida, R.; Muramatsu, M.; Miyamoto, H.; Igarashi, M.; Eguchi, N.; Sato, M.; et al. A single amino acid in the M1 protein responsible for the different pathogenic potentials of H5N1 highly pathogenic avian influenza virus strains. PLoS ONE 2015, 10, e0137989.
- Cheung, C.; Rayner, J.M.; Smith, G.J.; Wang, P.; Naipospos, T.S.P.; Zhang, J.; Yuen, K.-Y.; Webster, R.G.; Peiris, J.S.M.; Guan, Y.; et al. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 2006, 193, 1626–1629.
- Jiao, P.; Tian, G.; Li, Y.; Deng, G.; Jiang, Y.; Liu, C.; Liu, W.; Bu, Z.; Kawaoka, Y.; Chen, H. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J. Virol. 2008, 82, 1146–1154.
- Li, W.; Wang, G.; Zhang, H.; Xin, G.; Zhang, D.; Zeng, J.; Chen, X.; Xu, Y.; Cui, Y.; Li, K. Effects of NS1 variants of H5N1 influenza virus on interferon induction, TNFα response and p53 activity. Cell. Mol. Immunol. 2010, 7, 235–242.
- Dankar, S.K.; Wang, S.; Ping, J.; Forbes, N.E.; Keleta, L.; Li, Y.; Brown, E.G. Influenza a virus NS1 gene mutations F103L and M106I increase replication and virulence. Virol. J. 2011, 8, 13.
- Li, J.; Zhang, K.; Chen, Q.; Zhang, X.; Sun, Y.; Bi, Y.; Zhang, S.; Gu, J.; Li, J.; Liu, D.; et al. Three amino acid substitutions in the NS1 protein change the virus replication of H5N1 influenza virus in human cells. Virology 2018, 519, 64–73.
- Jonges, M.; Welkers, M.; Jeeninga, R.; Meijer, A.; Schneeberger, P.; Fouchier, R.; De Jong, M.; Koopmans, M. Emergence of the virulence-associated PB2 E627K substitution in a fatal human case of highly pathogenic avian influenza virus A(H7N7) infection as determined by illumina ultra-deep sequencing. J. Virol. 2013, 88, 1694–1702.
More
