Aconitic acid (propene-1,2,3-tricarboxylic acid) is the most prevalent 6-carbon organic acid that accumulates in sugarcane and sweet sorghum. As a top value-added chemical, aconitic acid may function as a chemical precursor or intermediate for high-value downstream industrial and biological applications. These downstream applications include use as a bio-based plasticizer, cross-linker, and the formation of valuable and multi-functional polyesters that have also been used in tissue engineering. Aconitic acid also plays various biological roles within cells as an intermediate in the tricarboxylic acid cycle (TCA) and in conferring unique survival advantages to some plants as an antifeedant, antifungal, and means of storing fixed pools of carbon. Aconitic acid has also been reported as a fermentation inhibitor, anti-inflammatory, and a potential nematicide.
- aconitic acid
- Microorganisms
- itaconic acid
- bio-based product
- TAA
- biological activity
- biological application
1. Introduction
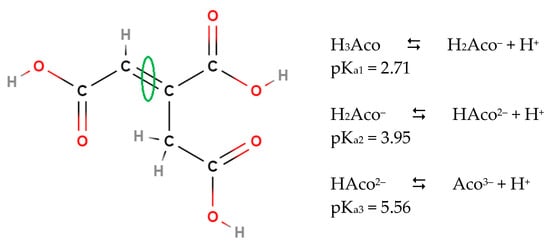
2. Microbial Conversion of Aconitic Acid to Itaconic Acid
3. Microbial Use as a Carbon Source
4. Aconitic Acid as a Fermentation Inhibitor
5. Nematocidal Activity of Trans-Aconitic Acid
6. Anti-Leishmanial Activity of Trans-Aconitic Acid
7. Aconitic Acid Production Confers Survival Advantages
7.1. Antifungal Defense
7.2. Antifeedant
7.3. Defense against Aluminum Toxicity
8. Biofilm Inhibition
9. Anti-Inflammatory Treatment
References
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass, Volume I: Results from Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwst National Laboratory and the National Renewable Energy Laboratory: Washington, DC, USA, 2004.
- Kanitkar, A.; Aita, G.; Madsen, L. The recovery of polymerization grade aconitic acid from sugarcane molasses. J. Chem. Technol. Biotechnol. 2013, 88, 2188–2192.
- King, W.D.; Kester, D.R. A general approach for calculating polyprotic acid speciation and buffer capacity. J. Chem. Educ. 1990, 67, 932–933.
- Pfendt, L.; Drazic, B.; Popovic, G.; Drakulic, B.; Vitnik, Z.; Juranic, I. Determination of all pKa values of some di- and tri-carboxylic unsaturated and epoxy acids and their polylinear correlation with the carboxylic group atomic charges. J. Chem. Res. (S) 2003, 2003, 247–248.
- Chun, H.L.; Lee, S.Y.; Lee, S.H.; Lee, C.S.; Park, H.H. Enzymatic reaction mechanism of cis-aconitate decarboxylase based on the crystal structure of IRG1 from Bacillus subtilis. Sci. Rep. 2020, 10, 11305.
- Kanamasa, S.; Dwiarti, L.; Okabe, M.; Park, E.Y. Cloning and functional characterization of the cis-aconitic acid decarboxylase (CAD) gene from Aspergillus terreus. Appl. Microbiol. Biotechnol. 2008, 80, 223–229.
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606.
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295.
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Frontiers in Microbiology 2013, 4, 23.
- Li, A.; van Luijk, N.; ter Beek, M.; Caspers, M.; Punt, P.; van der Werf, M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet. Biol. 2011, 48, 602–611.
- Deng, S.; Dai, Z.; Swita, M.; Pomraning, K.R.; Hofstad, B.; Panisko, E.; Baker, S.; Magnuson, J. Deletion analysis of the itaconic acid biosynthesis gene cluster components in Aspergillus pseudoterreus ATCC32359. Appl. Microbiol. Biotechnol. 2020, 104, 3981–3992.
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126.
- Steiger, M.G.; Punt, P.J.; Ram, A.F.J.; Mattanovich, D.; Sauer, M. Characterizing MttA as a mitochondrial cis-aconitic acid transporter by metabolic engineering. Metab. Eng. 2016, 35, 95–104.
- Zambanini, T.; Hartmann, S.K.; Schmitz, L.M.; Büttner, L.; Hosseinpour Tehrani, H.; Geiser, E.; Beudels, M.; Venc, D.; Wandrey, G.; Büchs, J.; et al. Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fung. Biol. Biotechnol. 2017, 4, 11.
- Yuhara, K.; Yonehara, H.; Hattori, T.; Kobayashi, K.; Kirimura, K. Enzymatic characterization and gene identification of aconitate isomerase, an enzyme involved in assimilation of trans-aconitic acid, from Pseudomonas sp. WU-0701. FEBS J. 2015, 282, 4257–4267.
- Rampazzo, P.E.; Marcos, F.C.C.; Cipriano, M.A.P.; Marchiori, P.E.R.; Freitas, S.S.; Machado, E.C.; Nascimento, L.C.; Brocchi, M.; Ribeiro, R.V. Rhizobacteria improve sugarcane growth and photosynthesis under well-watered conditions. Ann. Appl. Biol. 2018, 172, 309–320.
- Day, D.F.; Sarkar, D. Fuel alcohol from sweet sorghum: Microbial aspects. Dev. Ind. Microbiol. 1982, 23, 361–366.
- Wu, X.; Staggenborg, S.; Propheter, J.L.; Rooney, W.L.; Yu, J.; Wang, D. Features of sweet sorghum juice and their performance in ethanol fermentation. Ind. Crops Prod. 2010, 31, 164–170.
- Gibbons, W.R.; Westby, C.A. Cofermentation of sweet sorghum juice and grain for production of fuel ethanol and distillers’ wet grain. Biomass 1989, 18, 43–57.
- Amorim, H.V. Challenges to produce ethanol from sweet sorghum in Brazil. In Proceedings of the Sweet Sorghum Association 2015 Annual Conference, Orlando, FL, USA, 27–29 January 2015.
- Klasson, K.T. The inhibitory effects of aconitic acid on bioethanol production. Sugar Tech 2018, 20, 88–94.
- Klasson, K.T. Impact of potential fermentation inhibitors present in sweet sorghum sugar solutions. Sugar Tech 2017, 19, 95–101.
- Ghaemi, R.; Pourjam, E.; Safaie, N.; Verstraeten, B.; Mahmoudi, S.B.; Mehrabi, R.; De Meyer, T.; Kyndt, T. Molecular insights into the compatible and incompatible interactions between sugar beet and the beet cyst nematode. BMC Plant Biol. 2020, 20, 483.
- Du, C.; Cao, S.; Shi, X.; Nie, X.; Zheng, J.; Deng, Y.; Ruan, L.; Peng, D.; Sun, M. Genetic and biochemical characterization of a gene operon for trans-aconitic acid, a novel nematicide from Bacillus thuringiensis. J. Biol. Chem. 2017, 292, 3517–3530.
- Abad, P.; Gouzy, J.; Aury, J.-M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915.
- Misra, S.; Sanyal, T.; Sarkar, D.; Bhattacharya, P.K.; Ghosh, D.K. Evaluation of antileishmanial activity of trans-aconitic acid. Biochem. Med. Metab. Biol. 1989, 42, 171–178.
- Kar, S.; Kar, K.; Bhattacharya, P.K.; Ghosh, D.K. Experimental visceral leishmaniasis: Role of trans-aconitic acid in combined chemotherapy. Antimicrob. Agents Chemother. 1993, 37, 2459–2465.
- Gawron, O.; Jones, L. Structural basis for aconitase activity inactivation by butanedione and binding of substrates and inhibitors. Biochimica Biophysica Acta (BBA) Enzymol. 1977, 484, 453–464.
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42.
- Stout, P.R.; Brownell, J.; Burau, R.G. Occurrences of trans-aconitate in range forage species. Agron. J. 1967, 59, 21–24.
- Nelson, E.K.; Mottern, H.H. Some organic acids in barley, maize, oats and rye plants. J. Am. Chem. Soc. 1931, 53, 3046–3048.
- Igamberdiev, A.U.; Eprintsev, A.T. Organic acids: The pools of fixed carbon involved in redox regulation and energy balance in higher plants. Front. Plant Sci. 2016, 7, 1042.
- Cai, H.; Strouse, J.; Dumlao, D.; Jung, M.E.; Clarke, S. Distinct reactions catalyzed by bacterial and yeast trans-aconitate methyltransferases. Biochemistry 2001, 40, 2210–2219.
- Cai, H.; Dumlao, D.; Katz, J.E.; Clarke, S. Identification of the gene and characterization of the activity of the trans-aconitate methyltransferase from Saccharomyces cerevisiae. Biochemistry 2001, 40, 13699–13709.
- Sugimoto, T.; Kato, T.; Park, E.Y. Functional analysis of cis-aconitate decarboxylase and trans-aconitate metabolism in riboflavin-producing filamentous Ashbya gossypii. J. Biosci. Bioeng. 2014, 117, 563–568.
- Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Aconitate and methyl aconitate are modulated by silicon in powdery mildew-infected wheat plants. J. Plant Physiol. 2009, 166, 1413–1422.
- Orioli, G.A.; Thompson, J.F. Aconitate accumulation in wheat seedlings. Botanical Gazette 1990, 151, 30–37.
- Lv, J.; Xiao, J.; Guo, Z.; Dong, K.; Dong, Y. Nitrogen supply and intercropping control of Fusarium wilt in faba bean depend on organic acids exuded from the roots. Sci. Rep. 2021, 11, 9589.
- Kim, M.; Hen-Sik, K.; Tokio, O.; Hiroshi, F.; Shoziro, S. Isolation and identification of trans-aconitic acid as the antifeedant in barnyard grass against the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl. Entomol. Zool. 1976, 11, 53–57.
- Nagata, T.; Hayakawa, T. Antifeeding activity of aconitic acids and oxalic acid on brown planthopper, Nilaparvata lugens (Stål) and green rice leafhopper, Nephotettix cincticeps (Uhler). Jap. J. Appl. Entomol. Zool. 1998, 42, 115–121.
- Watanabe, K.; Katsuharar, M.; Nakao, H.; Sato, M. Detection and molecular analysis of plant- and insect-associated bacteria harboring aconitate isomerase involved in biosynthesis of trans-aconitic acid as antifeedant in brown planthoppers. Curr. Microbiol. 1997, 35, 97–102.
- Hattori, M. Probing behavior of the brown planthopper, Nilaparvata lugens Stål (Homoptera: Delphacidae) on a non-host barnyard grass, and resistant and susceptible varieties of rice. Appl. Entomol. Zool. 2001, 36, 83–89.
- Rustamani, M.A.; Kanehisa, K.; Tsumuki, H. Aconitic acid content of some cereals and its effect on aphids. Appl. Entomol. Zool. 1992, 27, 79–87.
- Uchimiya, M.; Knoll, J.E. Rapid data analytics to relate sugarcane aphid population and damage on sorghum (Sorghum bicolor (L.) Moench). Sci. Rep. 2019, 9, 370.
- Knoll, J.E.; Uchimiya, M.; Harris-Shultz, K. Juice chemical properties of 24 sorghum cultivars under varying levels of sugarcane aphid (Melanaphis sacchari) infestation. Arthropod-Plant Interact. 2021, 15, 707–719.
- Udén, P. Plant organic acids in fresh and ensiled forage plants. Grass Forage Sci. 2018, 73, 583–587.
- Pintro, J.; Barloy, J.; Fallavier, P. Effects of low aluminum activity in nutrient solutions on the organic acid concentrations in maize plants. J. Plant Nutr. 1997, 20, 601–611.
- Bortolo, T.D.S.C.; Marchiosi, R.; Viganó, J.; Siqueira-Soares, R.D.C.; Ferro, A.P.; Barreto, G.E.; Bido, G.D.S.; Abrahão, J.; dos Santos, W.D.; Ferrarese-Filho, O. Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max. Plant Physiol. Biochem. 2018, 132, 490–496.
- Pestana-Nobles, R.; Leyva-Rojas, J.A.; Yosa, J. Searching hit potential antimicrobials in natural compounds space against biofilm formation. Molecules 2020, 25, 5334.
- Antoniani, D.; Bocci, P.; Maciag, A.; Raffaelli, N.; Landini, P. Monitoring of diguanylate cyclase activity and of cyclic-di-GMP biosynthesis by whole-cell assays suitable for high-throughput screening of biofilm inhibitors. Appl. Microbiol. Biotechnol. 2010, 85, 1095–1104.
- Cho, K.H.; Tryon, R.G.; Kim, J.H. Screening for diguanylate cyclase (DGC) inhibitors mitigating bacterial biofilm formation. Front. Chem. 2020, 8, 264.
- Oliveira, D.P.; Moreira, T.D.V.; Batista, N.V.; Souza Filho, J.D.; Amaral, F.A.; Teixeira, M.M.; Pádua, R.M.; Braga, F.C. Esterification of trans-aconitic acid improves its anti-inflammatory activity in LPS-induced acute arthritis. Biomed Pharmacother 2018, 99, 87–95.
- Pinto de Oliveira, D.; Guimarães Augusto, G.; Vieira Batista, N.; de Oliveira, V.L.S.; Santos Ferreira, D.; Castro, E.S.M.A.; Fernandes, C.; Almeida Amaral, F.; Martins Teixeira, M.; Maia de Pádua, R.; et al. Encapsulation of trans-aconitic acid in mucoadhesive microspheres prolongs the anti-inflammatory effect in LPS-induced acute arthritis. Eur. J. Pharm. Sci. 2018, 119, 112–120.
- Garcia, E.D.F.; De Oliveira, M.A.; Dourado, L.P.A.; De Souza, D.G.; Teixeira, M.M.; Braga, F.C. In vitro TNF- α inhibition elicited by extracts from Echinodorus grandiflorus leaves and correlation with their phytochemical composition. Planta Med. 2016, 82, 337–343.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 1997, 23, 3–25.
