Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Niraj Singh Tharu and Version 2 by Rita Xu.
Spinal cord injury (SCI) is one of the most debilitating injuries in the world. Complications after SCI, such as respiratory issues, bowel/bladder incontinency, pressure ulcers, autonomic dysreflexia, spasticity, pain, etc., lead to immense suffering, a remarkable reduction in life expectancy, and even premature death. Traditional rehabilitations for people with SCI are often insignificant or ineffective due to the severity and complexity of the injury. Two most promising noninvasive spinal cord electrical stimulation methods of SCI rehabilitation treatment, namely, trans-spinal direct current stimulation (tsDCS) and trans-spinal pulsed current stimulation (tsPCS).
- spinal cord injury
- rehabilitation
- neuromodulation
1. Introduction
Spinal cord injury (SCI) is sudden, devastating, debilitating, and life-altering neurological damage that is correlated with severe physical, mental, social, and vocational impacts on the individual, family members, and healthcare systems [1][2][3][4][5][1,2,3,4,5]. Worldwide, the estimated incidence of SCI is between 250,000 and 500,000 per year [3]. Damage to the spinal cord can be permanent, which may result in either tetraplegia or paraplegia that may lead to functional impairment [5]. The American Spinal Injury Association (ASIA) Impairment Scale (AIS) classifies SCI into two categories: either complete or incomplete. AIS-A is defined as no sensory and motor function in the lowest sacral segment (S4–S5). In contrast, in incomplete (AIS-B, C, D, or E) SCI, there is some sensory and motor preservation as far as the lowest sacral segment (S4–S5) [6][7][8][6,7,8]. An individual with an SCI is at risk of different types of secondary complications which leads to premature death in most of the cases [9]. Secondary complications, such as pressure ulcers, spasticity, pain, urinary tract infections, depression, bowel problems, cardiovascular complications, renal stones, autonomic dysreflexia, fracture [10], fatigue, respiratory complications, autonomic dysreflexia, and bladder dysfunction, hamper the healthy wellbeing, social participation, and productive activities as well as quality of life [11]. Among all the complications, paralysis or reduced mobility are the most visible complications after SCI [1]. Reversing the paralysis often reduces the secondary complications and, thus, improves the quality of life of these individuals with SCI [12]. Currently, there is no standard neuromodulatory treatment for spinal cord injury (SCI). Conventional treatment approaches aim to reduce secondary complications and improve the functions that have been retained after the injury [13]. However, in recent years, various neuromodulatory approaches, such as epidural spinal cord stimulation (SCS) [14], intraspinal microstimulation, functional electrical stimulation (FES) [15] and transcutaneous spinal cord stimulation (tSCS) [16], have been studied extensively. Among them, noninvasive stimulations such as FES and tSCS are comparatively safer [17] and easier to implement in clinical settings than the invasive ones [18]. Noninvasive tSCS is a relatively new technology which targets the neural structure similar to its invasive counterpart, SCS [19]. However, depending on the stimulation parameters, tSCS can be broadly divided into two types: direct current stimulation (tsDCS) and pulse current stimulation (tsPCS).
1.1. Conventional Treatments and Management after SCI
Current SCI rehabilitation depends on the injury and impairment level which concentrates on the most appropriate recovery from injury and helps a person to return to the community in a maximally independent way. This long, time-consuming rehabilitation process includes bed mobility practice, wheelchair propel training, strengthening programmes, range of motion exercises, passive movements, stretching exercises, bowel/bladder function management, and a higher level of functional activity [20][21][20,21].
Conventional rehabilitation is an undistinguishable, weak treatment protocol which has very few guidelines. Although rehabilitation scientists have covered some parts, there is still no universal guideline [12]. Generally, clinicians continuously concentrate on the emergence-based treatment for independence and planning of the discharge of patients as quickly as possible because of the limitation of inpatient support and funding. Conventional acute rehabilitation, however, may vary from area to area or even practitioner to practitioner [12][22][12,22].
Respiratory problems are one of the major secondary complications after cervical cord injury, where muscle becomes impaired which leads to a decreased vital capacity and coughing ability. Therefore, they turn into secondary complications such as pneumonia which is one of the major causes of premature death after cervical SCI. Conventional rehabilitation for pulmonary function improvement involves a combination of management such as positioning, breathing exercises, respiratory muscle strengthening, etc. [12]. From the physical and psychological perspectives, neurogenic bowel/bladder dysfunction is a major secondary complication for a person with SCI. The timely cleaning of catheters, regular intake of food containing fibre, adequate drinking of water, and the manual evacuation or use of suppository and oral medication are some regular routines in conventional bladder/bowel management [23]. Spasticity and pain are other common secondary complications after SCI. Severe spasticity may lead to decreased mobility, decreased activity of daily living, the development of contractures, and pressure ulcers. Passive stretching, weight-bearing exercises, positioning, and oral medications can minimize spasticity [24]. More than 80% of people suffer from pain after SCI resulting in emotional disturbance, decreasing community participation and finally the hampering of the quality of life. The active range of movement, passive stretching, hot compression, and oral medications are used to decrease the severity of pain [25].
Autonomic dysreflexia (AD)—a potentially life-threatening illness—is frequently caused by a spinal cord segment. Patients with SCI at the T6 level or higher may experience autonomic dysreflexia [26][27][26,27]. Resting blood pressure after SCI is frequently lower than usual (hypotension), while a sudden increase in blood pressure of as little as 20 mm Hg can cause life-threatening emergencies [28]. This hypertension, which is a symptom of a disorder known as autonomic dysreflexia (AD), affects 50–90% of SCI patients with tetraplegia or high paraplegia [26]. When it occurs, sitting in an upright position, taking off any restrictive clothes, socks and shoes, immediate catheterization, medication for decreasing blood pressure, etc., need to be performed in an urgent manner for AD management [26].
Fatigue is a complex condition that involves physical, behavioural, and psychological processes, and is a factor that is responsible for depression and self-resilience. It is also a common symptom for a person with SCI. Fatigue is also associated with decreasing positive feelings and responses to physical tiredness resulting in anxiety, decreased cognition, cardiac arrhythmia, and functional inactivity. Previous research works suggested that the reduction in poor social interactions can improve psychomotor and vocational functioning [29][30][29,30].
1.2. Functional Electrical Stimulation (FES) Therapy for SCI Rehabilitation
Functional electrical stimulation (FES) is a neurorehabilitation technology that applies short pulses of electrical stimulation during specific mimicking activities such as walking or cycling [15][31][15,31]. It can be applied in different areas of the body through the skin above the targeted muscle and peripheral nerve. It is more preferred to apply FES over the target muscle fibres rather than nerves as there is less chance of tissue damage and also achieves a higher success rate but with less electrical power [32]. FES pulses are primarily quantified according to three parameters, including pulse width, frequency, and amplitude. Square waveform patterns and 300 to 600-microsecond pulse width waveforms are generally applied to exert different effects on the targeted muscle. The frequency of the stimulation varies depending on the specific purpose. Normally, it ranges from 20 to 50 Hz, where a low frequency is usually applied to avoid muscle fatigue and to induce smooth muscle contraction with a tingling but comfortable feeling. The usual range of amplitude of FES is a few milliamperes to 100 mA, where a high amplitude has more potential to achieve a depolarizing effect leading to the activation of more muscle fibres. The range of amplitude also depends on the time and shape of the stimulation as well as the applied area of the body part [32]. There are numerous benefits of FES therapy in SCI rehabilitation, including the enhancement of residual muscle strength, increased flexibility and range of motion of the joints or limbs, as well as a reduction in spasticity, which leads to enhanced sensorimotor functions [32]. FES has a wide range of applications in rehabilitation, as it has both direct and indirect effects [30]. FES mostly enhances and brings back upper and lower limb muscle functions [31][32][31,32], improves cardiopulmonary fitness such as rising peak ventilation, thereby enhancing the airflow rate and airway pressure [33], and decreases neuropathic or nociceptive pain and spasticity which commonly arise after SCI [34]. Pressure ulcers are another common complication after SCI, where FES plays an important role to increase the pressure ulcer healing rate [35]. In addition, FES helps to control bowel/bladder dysfunction as well as improve erection and ejaculation dysfunction [36]. Furthermore, it was found that FES increases circulation and body metabolism, which ultimately results in an improved muscle mass and balance, and a controlled posture [36].
1.3. Spinal Cord Stimulation (SCS) Therapy for SCI Rehabilitation
Epidural spinal cord stimulation (SCS) is an effective neuromodulatory treatment which reduces chronic pain, reduces the severity of spasticity, increases specific rhythmic motor activity in the lower limbs, activates the respiratory muscles, improves bladder control, and enhances sensory nerve activity and the capacity to affect numerous organs either in the autonomic nervous system or viscero-somatic reflexes [14][37][38][39][14,37,38,39]. This invasive stimulation technique is a challenging innovation because it needs comprehensive education, training, and pre- and post-operative patient cooperation.
A few additional key points must be considered before SCS surgery, including if the patient needs any kind of antithrombotic therapy, suffers from coagulopathy, or has any kind of infection. Furthermore, all participants should be assessed regarding the cause of any psychiatric or psychological problem which can interrupt the effectiveness of an epidural SCS [40]. An epidural SCS can also experience some technical and clinical complications. The technical complications include the breakdown of devices, current leakage, and malfunction of rechargeable batteries, which can lead to failure [40]. In general, complications arise from an epidural SCS at a staggeringly high rate of about 28% to 42% [41]. The technical complication rate of the devices is around 5% [42]. Clinical complications include tissue damage, bleeding, and haematoma, plus infection, which is the most common (4% to 10%) [41][43][44][41,43,44].
To harness the benefits and to avoid the complications of an epidural SCS, a noninvasive spinal cord neuromodulation modality, called transcutaneous SCS, has been developed and investigated recently [45][46][47][48][45,46,47,48]. Transcutaneous stimulation can be induced alone or in combination with functional therapy, which leads to an increased motor function in patients with chronic paralysis [17][49][17,49]. It has the therapeutic potentiality to improve voluntary motor function, improve upper and lower motor limb muscle strength, improve standing posture, improve gait, decrease spasticity, and improve trunk strength and overall spinal function [50][51][52][50,51,52]. Usually, the electrodes are placed on the skin over the spinous processes of the vertebral column [18]. Transcutaneous SCS can modulate the spinal reflex of the lower limbs and activate the sensory–motor fibres of the anterior/posterior root [18]. Additionally, in the lower limbs, it can stimulate multiple muscles at the same time [46]. It is a safe therapeutic tool which is also appropriate for all SCI patients except those with skin irritation [17].
2. Trans-Spinal Direct Current Stimulation (tsDCS)
Direct current (DC) stimulation is a noninvasive tool of neuromodulation which applies a simple, safe, effective, and painless constant stimulation [16][53][54][55][56][16,53,54,55,56]. DC stimulation can influence a change in the cortical and subcortical transmission of the spinal cord, resulting in a functional improvement after SCI. The impact of DC stimulation depends on the connected field related to the underlying neuron, neuronal junction, and polarization of electrodes such as anodal and cathodal stimulation [57].
Trans-spinal direct current stimulation (tsDCS) uses DC with a constant intensity during neuromodulation. tsDCS electrodes are generally thick (6 mm), rectangular (7 × 5), and have an alike longitudinal pair of saline-soaked synthetic sponges enclosed by electrolyte gel [16][54][55][56][58][16,54,55,56,58]. These electrical fields need to be applied directly over the spine [53][55][56][59][53,55,56,59]. The electrical current is sourced from a battery-driven steady current stimulator (Figure 1). Generally, two electrodes are positioned in different areas of the body: one is placed above the targeted area of the spinous process of the vertebra and the other is commonly applied over the posterior part of the limbs or shoulders. tsDCS consistently delivers a continual current of 1.5 mA to 2.5 mA for about 15–20 min [54][55][56][59][54,55,56,59]. The current density of 0.06 mA/cm2 and total charge density of 0.064 C/cm2 [16][59][16,59] are sufficiently low enough to avoid any potential tissue damage [58]. The current steps up to the maximal level within 10 s and in the same way decreases at the end of the treatment session [54]. It induces changes in spinal cord physiology, nociceptive pathways, and cortical excitability, as well in the supraspinal centres [53][54][53,54]. On the other hand, large dissimilarities can be observed according to the participant’s physiology and anatomical parameters, such as age, sex, height, and weight [60][61][60,61]. The use of tsDCS is limited due to the large range of electrical currents that can be used in clinical settings [16] and the location of the electrodes, as even a 1 cm displacement can have a significant impact on the projected current flow pattern [62]. Headaches, weariness, vasodilation generating erythema, tingling, and itchiness are frequently reported side-effects [62].
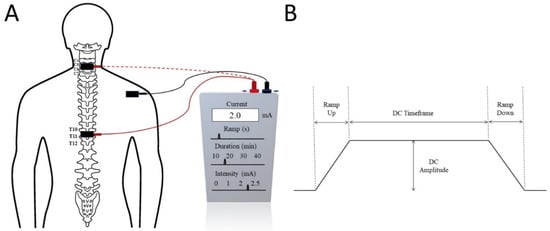

Figure 1. (A) Trans-spinal direct current stimulation (tsDCS) at the cervical (dotted red) or thoracic (solid red) spinal level with the reference electrode commonly placed on the shoulder. (B) Typical tsDCS signal with several seconds of ramp up and ramp down current. The treatment intensity and duration are adjusted by the DC Amplitude and DC Timeframe.
2.1. Mechanism of tsDCS
tsDCS is one of the neuromodulatory tools which work directly on the neurons and have long-term effects on the spinal cord [57]. The exact mechanism of tsDCS is not fully understood, but a few researchers found that tsDCS on resting membranes acts equally for long-term potentiation and long-term depression. Moreover, tsDCS also causes a change to glutamatergic neurotransmission (a chemical that nerve cells use to send signals to other cells) [63]. Glutamate facilitates the pathway of phrenic motor neurons which provide complete motor innervations of the respiratory diaphragm, and consist of motor, sensory, and sympathetic nerve fibres, as well as N-methyl-D-aspartate (NMDA) receptors, which are very important for controlling synaptic plasticity and memory function [64]. Therefore, it leads to the neuronal activation process of the spinal cord. As a result, long-haul potentiation occurs in postsynaptic spinal cord motoneurons [58]. tsDCS stimulates neurotransmitters in the brain or in the spinal cord and also stimulates neural function through the ascending spinal pathways. Conductive elements of the corticospinal tract change after tsDCS; therefore, the resting motor threshold also changes [55]. tsDCS interacts between two functional neural circuits [63], which modulates the inhibitory tone and could be a therapeutic tool to decrease spasticity [55]. tsDCS may also activate the supraspinal loops which are transmitted by the brainstem or thalamo-cortical frameworks, followed by both ascending and descending tract inhibition [65].
Inactive motor neurons are activated after the use of tsDCS, which improves the connectivity between two functional neural circuits and a remarkable change comes about through the spinal motor neurons [66]. This helps to promote the inhibitory tone which is responsible for decreasing the severity of spasticity resulting from SCI. Additionally, tsDCS depends on the polarity of the stimulation, in relation to which researchers have found that cathodal stimulation activates neurons, whereas anodal stimulation depresses neurons [67]. With the use of anodal tsDCS stimulation, the nociceptive pathway decreases its conductivity and, subsequently, balances somatosensory-evoked possibilities which results in a decrease in pain sensations [68][69][68,69].
2.2. tsDCS in SCI Rehabilitation
In SCI rehabilitation, tsDCS has a significant implication on the promotion of axonal regeneration and is also responsible for preventing fibre degeneration [70]. Chronic SCI causes automatic micturition and neurogenic detrusor overactivity in both animals and humans. Following SCI, synchronized activity is lost, resulting in detrusor sphincter dyssynergia and inadequate bladder discharge, urological complications such as urinary tract infections, and chronic renal failure as a result of this process [71][72][71,72]. A cathode tsDCS has a significantly altered effect on sacral spinal reflexes and spinal neuronal networks, as well as bladder and external urinary sphincter functions in mice with an intact spinal cord and SCI [73][74][73,74]. Furthermore, tsDCS is an important neuromodulation therapeutic intervention which has been found to be safe, and no harmful effects on spinal-specific or other parts of the body have been observed after stimulation [70].
More than 80% of SCI patients have reported that they had been suffering from pain and 70% reported their suffering from spasticity after injury which had hampered their ability to partake in activities of daily living [11][75][76][11,75,76]. tsDCS is a simple and low-cost neuromodulation technique used in rehabilitation after neurological injury [16]. The Hoffman reflex (H-reflex) is usually utilized as a test to examine spinal inhibitory intraneuronal circuits. There is a relation between the changing of the sensitivity of the H-reflex and improving new motor function as well as new motor skills [54]. Albuquerque et al. and Awosika et al. [53][54][53,54] used tsDCS combined with motor training to decrease plasticity and maximize motor recovery for patients with neurological problems. Bocci et al. and Hubli et al. [56][77][56,77] worked with tsDCS by combining it with locomotion, where they found an improvement in gait after the use of tsDCS. The rhythmic activity of the brainstem central pattern generator leads to an increase in respiratory muscle contraction and duration; although tsDCS cannot increase the inspiratory time, it can increase the tidal volume [58]. tsDCS can facilitate cortical sensitivity and has been suggested as a promising solution for the comprehensive extension of neurological and psychiatric disorders [63]. It also helps to decrease pain severity, increase pain tolerance [55][56][55,56], reduce spasticity, and promote functional movements [60][61][64][60,61,64] (Kuck) [66].
