Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. It is principally associated with liver cirrhosis and chronic liver disease. The major risk factors for the development of HCC include viral infections (HBV, HCV), alcoholic liver disease (ALD,) and non-alcoholic fatty liver disease (NAFLD). The optimal treatment choice is dictated by multiple variables such as tumor burden, liver function, and patient’s health status. Surgical resection, transplantation, ablation, transarterial chemoembolization (TACE), and systemic therapy are potentially useful treatment strategies. TACE is considered the first-line treatment for patients with intermediate stage HCC. The purpose of this entry was to assess the indications, the optimal treatment schedule, the technical factors associated with TACE, and the overall application of TACE as a personalized treatment for HCC.
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy, representing approximately 90% of primary liver cancers
[1]. HCC constitutes a major health problem with an increasing incidence over the years in both developed and developing countries
[1]. HCC is principally associated with liver cirrhosis and chronic liver disease. Approximately, one third of cirrhotic patients will develop HCC eventually in their lifetime, with a 1-year rate of 1–8%
[2]. The major risk factors for the development of HCC are viral infections (hepatitis B and C virus-HBV, HCV), alcoholic liver disease (ALD), and non-alcoholic fatty liver disease (NAFLD). Viral hepatitis represents the most common risk factor for HCC. Nevertheless, vaccination for HBV and antiviral therapy for HBV/HCV have reduced the incidence of HCC in countries with an organized vaccination program, while NAFLD-related cirrhosis continues to increase, representing the leading cause of HCC in the developed world
[3].
HCC is usually diagnosed during routine examination, since most cirrhotic patients in developed countries enter a screening program. Unfortunately, many countries do not have an organized screening program and HCC patients are often diagnosed in advanced stage. All high-risk patients for HCC should be monitored with ultrasonography (US) and alpha-fetoprotein (AFP) measurement every 6 months
[1]. Nevertheless, since US is considered operator-dependent and AFP is often normal in early stage, computed tomography (CT) and magnetic resonance imaging (MRI) are used to characterize nodules bigger than 10 mm.
Over the years, several HCC classification systems have been developed. Most of them include parameters such as tumor stage, liver function impairment, patient’s performance status, and recommended treatment strategy. The Barcelona Clinic Liver Classification (BCLC) is a staging system widely accepted worldwide
[4]. BCLC stratifies patients according to the natural history of the disease, selecting the best candidates for the best therapies
[1][5][1,5].
2. Treatment Procedure
TACE treatment involves the infusion of highly concentrated dose of chemotherapy through selective catheterization of the arterial branch feeding the tumor. The embolization of the tumor microcirculation following the infusion results in prolonged cytotoxic effect, minimizing the systemic toxicity of chemotherapy
[6][14]. The dual blood supply to the liver, from the hepatic artery and the portal vein, makes TACE, as well as arterially directed therapies in general, possible, and protects healthy liver tissue from ischemia. on the contrary to the normal parenchyma that derives blood supply mostly from the portal vein, tumor cells get blood flow mainly from the hepatic artery
[7][15].
2.1. cTACE vs. DEB-TACE
There are two types of TACE techniques: conventional TACE (cTACE) and TACE with drug-eluting beads (DEB-TACE). cTACE uses a cytotoxic agent such as doxorubicin, epirubicin, mitomycin, or cisplatin, followed by the infusion of Lipiodol, an oily radio-opaque agent, as a chemotherapeutic carrier, as well as an embolic material
[8][16]. Other embolic agents commonly used are degradable starch microspheres (DSM), collagen, and gelatine sponge (Gel-foam). DEB-TACE uses non-resorbable embolic microspheres loaded with chemotherapy drugs that are capable of releasing the agent in a sustained manner
[9][17].
It is still controversial whether the one technique is superior to the other. In a meta-analysis performed by Zou et al., DEB-TACE appeared to have an improved complete response rate and overall survival rate when compared to cTACE. Furthermore, DEB-TACE patients reported decreased common adverse events than cTACE, with no statistically important difference between the two therapies on serious adverse events
[10][18]. Chen et al. and Han et al. reached a similar conclusion; the overall survival rates were significantly higher in the DEB-TACE group, with no statistically significant difference in tumor response and treatment-related adverse events
[11][12][19,20]. On the contrary, two meta-analyses concluded that cTACE and DEB-TACE had similar therapeutic results, overall survival, and adverse events rates, underlying the need for further research with high-quality studies
[13][14][21,22].
Recently, a new method of arterial occlusion during TACE has been proposed
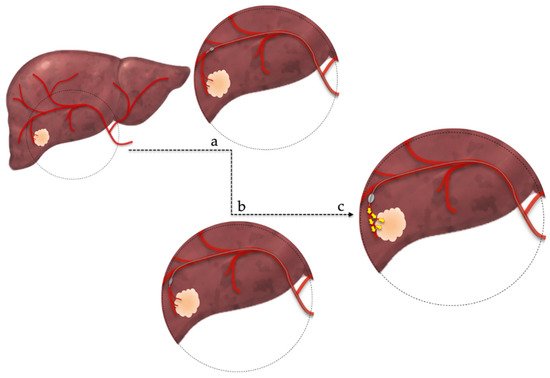
[15][23]. This new method, named balloon-occluded transarterial chemoembolization (B-TACE), uses a balloon micro-catheter in order to selectively occlude the arterial micro-circulation of the tumor (
Figure 1)
[16][17][24,25]. The advantages of this method are the prevention of embolic agents’ leakage and the increased accumulation of Lipiodol emulsion within the tumor that may enhance treatment success due to prolonged cytotoxic effect
[15][23]. The current literature suggests a potential advantage of B-TACE, compared to DEB-TACE, for patients with large tumors, but further studies must be conducted to reach safe conclusions
[18][26].
Figure 1. Balloon-occluded transarterial chemoembolization technique. (a,b): superselective catheterization of the arterial branch feeding the tumor, (c): occlusion of feeding artery by infletion of a microballoon catheter and subsequent administration of chemotherapeutic regimen.
2.2. Patient Selection
In accordance with the BCLC guidelines, TACE is currently considered the first-line treatment for selected patients with HCC in the intermediate stage (BCLC stage B). This stage includes patients with unresectable, multinodular tumors without vascular invasion or extrahepatic spread.Moreover, these patients present heterogeneous features, in terms of tumor burden and liver function (Child-Pugh A or B)
[19][27]. Therefore, not all intermediate-stage HCC patients benefit the same from TACE, since this heterogeneity makes the behavior of the tumor difficult to predict. Various systems for subclassification of intermediate-stage HCC have been proposed. Among these systems, up-to-seven criteria, originally used to predict the prognosis of HCC patients undergoing LT, were also proposed to subclassify patients within BCLC-B stage
[20][28]. These criteria include the sum of the diameter of the largest tumor (in cm) and the number of tumors
[21][29].
TACE can also be beneficial for some patients beyond BCLC stage B. In patients with early stage disease (BCLC A) who are unsuitable for surgery or locoregional ablation, TACE consists a safe and effective option with a high response rate and very good outcomes
[22][31]. TACE can also be performed prior to liver transplantation as a bridging treatment while the patient is on the waiting list or as downstaging treatment to within Milan criteria
[8][16]. Finally, some patients with advanced disease can be treated with TACE. In those patients, TACE is possible if they have segmental or sub-segmental portal vein thrombosis and the treatment is selective. A recent meta-analysis showed improved overall survival in the TACE group and better tumor response when compared with conservative treatment
[23][32].
- 2.3. Contraindications and Adverse Effects
There are limited contraindications to TACE therapy, mainly concerning the residual liver function or impaired portal blood flow. Advanced cirrhosis (Child-Pugh C), liver failure, total bilirubin > 3 mg/dL, presence of extrahepatic disease, complete portal vein thrombosis, uncorrectable coagulopathy, and the presence of high-flow arterioportal or arteriovenous shunts represent some of them
[19][27]. Severe atherosclerotic disease, renal insufficiency, and allergy to contrast material are considered relative contraindications
[24][34].
Even though TACE is considered a relatively safe procedure, several adverse events have been documented. Since the hepatic artery also supplies the biliary plexus, TACE can cause ischemic complications such us pancreatits, cholecystitis, andbile duct necrosis, but also liver and biliary injuries, liver abscess formation, and less selective embolization resulting in liver failure
[7][15].The TACE-related mortality rate is considered low (<1%)
[25][35]. Most commonly, up to 47% of patients treated with TACE develop a clinical syndrome mediated by an inflammatory response, as a result to cytokines’ release. This post-embolization syndrome (PES) presents with fever, right upper quadrant abdominal pain, and nausea with or without vomiting
[26][36]. PES is associated with prolonged hospital stays and recurrent admissions, but it is also considered an early predictor of worse overall survival
[26][27][36,37]. Prophylactic administration of steroids and 5-HT3 receptor antagonists has been used to prevent PES
[28][38]. In a retrospective study by Haohao et al., the lipiodol + dexamethasone emulsion significantly reduced the incidence rate of post-embolization syndrome
[29][39].
3. Prognostic Scores
Over the last decade, several prognostic scores have been developed to predict the results of TACE treatment. (
Table 1) The criteria for TACE refractoriness has been established for the first time in the world by the Japan Society of Hepatology (JSH) in 2011
[30][40]. TACE failure/refractoriness is defined by the International Expert Panel of Interventions in Hepatocellular Carcinoma (EPOIHCC) as no response after three or more TACE procedures within a period of six months
[31][41]. The incidence of TACE failure/refractoriness is considered to be quite high, ranging from 37 to 49.3%
[32][42]. Therefore, it is essential to identify prognostic factors in order to differentiate patients who would benefit or not from repeated TACE procedures.
Table 1. Prognostic scores. AFP: alpha-fetoprotein, TACE: transarterial chemoembolization, AST: aspartate protein, BCLC: Barcelona Clinic Liver Classification, HCC: Hepatocellular carcinoma.
|
Score
|
Parameters Used
|
Prognostic Value
|
Demerits
|
|
HAP [33][43]
|
Albumin, Bilirubin, AFP, Size of dominant tumor
|
Prognosis of HCC patients undergoing TACE
|
|
|
ART [34][44]
|
Radiological response after the first TACE, increase of serum AST, increase of Child-Pugh score
|
Differentiation of patients who would benefit from a second TACE
|
Failed to predict overall survival in patients who received repeated TACE
|
|
STATE [35][45]
|
Albumin, CRP, Size of the largest tumor, Number of tumors
|
Identification of patients unsuitable for first-time TACE
|
|
|
ABCR [36][46]
|
AFP, BCLC, Child-Pugh increase, Radiological response
|
Differentiation of patients who would benefit from a second TACE
|
Failed to show sufficient prognostic ability to guide the decision-making process regarding subsequent TACE
|
|
CHIP [37][47]
|
Child-Pugh, number of lesions, HCV-RNA positivity
|
Stratification of patients within BCLC Stage B
|
|
|
M-TACE [38][48]
|
Bilirubin, INR, CRP, creatinine, AFP, tumor extension
|
Identification of patients most likely to benefit from TACE
|
|
|
Six & Twelve [39][49]
|
Tumor size, tumor number
|
Outcome prediction and risk stratification of recommended TACE candidates
|
Only “ideal” TACE candidates included
|
|
Pre-TACE & Post-TACE predict [40][50]
|
Tumor size, tumor number, AFP, albumin, bilirubin, vascular invasion, cause, radiological response
|
Prediction of survival among patients receiving TACE
|
Calculator needed
|
The Hepatoma arterial-embolization prognostic (HAP) score stratifies patients into four groups. Patients are divided according to their albumin, bilirubin, AFP levels, and the size of dominant tumor. One point is assigned if albumin < 36 g/dL, bilirubin > 17 μmol/L, AFP > 400 ng/mL, or size of dominant tumor > 7 cm. The HAP score is calculated by the sum of these points, and patients are classified into low-(HAP A, score 0), intermediate-(HAP B, score 1), high-(HAP C, score 2), or very high-(HAP D, score > 2) risk groups. The median survival for the groups A, B, C, and D was 27.6, 18.5, 9.0, and 3.6 months, respectively
[33][43].
The ART score (Assessment for Retreatment with TACE) was created to differentiate patients who would benefit from a second TACE procedure. The creators of the score conducted a study by dividing patients in two groups based on radiologic tumor response after the first TACE, whether there was an increase of serum AST (aspartate aminotransferase) >25%, and whether there was an increase of Child-Pugh score of 1 or ≥2 points. The two groups (group one: ART score between 0–1.5 points, group two: ART score ≥ 2.5 points) showed significantly different median overall survivals (23.7 versus 6.6 months). Therefore, the study concluded that a higher ART score was associated with worse prognosis and major adverse events and that those patients may not benefit from further TACE
[34][44].
The STATE-score (Selection for TrAnsarterial chemoembolization TrEatment) was created to identify patients who are unsuitable for first-time TACE. Hucke et al. divided patients in two groups (<18, ≥18 points) according to their albumin and CRP levels and whether they are in or beyond the up-to seven-criteria (if the sum of the diameter of the largest tumor and the number of tumors is less than seven). The median survival was 5.3 months for the first group (<18 points) and 19.5 months for the second (≥18 points). The researchers concluded that a lower STATE-score was associated with increased mortality after TACE-1
[35][45]. They also combined the STATE and ART score, namely START strategy to identify the best candidates for multiple TACE
[35][45].
The ABCR score (Alpha-fetoprotein, BCLC, Child-Pugh, and Response), similarly to the ART score, was developed to identify appropriate patients who would benefit from TACE retreatment. This score, ranging from minus three to six, includes four parameters: AFP, BCLC, Child-Pugh increase by more than two points, and radiological response. The analysis conducted by Abhoute et al. in order to validate the score revealed that patients with ABCR score ≥4 after the first TACE procedure had a median overall survival < 5.1 months and probably would not benefit from repeated TACE
[36][46].
The CHIP score (Chiba HCC in Intermediate-stage Prognostic), helps stratify patients within the heterogeneous stage B. This score, with a range between zero and seven, is defined by the sum of three subscale scores: Child-Pugh, number of lesions, and HCV-RNA positivity. According to their sum, patients were stratified in five groups (zero to two points, three points, four points, five points, and six to seven points). The creators of the score came to the conclusion that each group corresponds to different prognosis (65.2, 29.2, 24.3, 13.1, and 8.4 months median OS, respectively)
[37][47].
The Munich-TACE score (M-TACE) uses the values of bilirubin, international normalized ratio, C-reactive protein, creatinine, and AFP, as well as tumor extension (size and number of nodules, vascular invasion, metastasis) to divide patients in three subgroups. M-TACE was validated in a cohort analysis revealing that patients in group one (zero to nine points) had a median survival of 35.2 months, patients in group two (10–13 points) had a median survival of 16.9 months and finally patients in group 3 (>13 points) had a median survival of 8.6 months
[38][48].
Recently, a novel prognostic score has been developed. This new stratification model, named ‘six and twelve’ score, divides patients in three groups, according to the sum of tumor size (diameter of the largest nodule) and tumor number (group one: sum ≤ 6, group two: 6 < sum ≤ 12, group three: sum > 12). The creators of the score conducted a validation analysis that resulted in distinct prognosis. The median survival rates for each group were 49.1 months, 32 months, and 15.8 months, respectively
[39][49]. In contrast to previous scores, this study included only “ideal” TACE candidates, defined as treatment-naïve patients, unresectable BLCL stage A or stage B, with Child-Pugh scores between A5 and B7 and performance status 0.
In 2020, Han et al. created two new prognostic scores: the pre-TACE model (“Pre-TACE-Predict”) and the post-TACE model (“Post-TACE-Predict”). The parameters included in point assigning were tumor number and size, alpha-fetoprotein, albumin, bilirubin, vascular invasion, cause, and response, as assessed by mRECIST criteria. According to their score, patients were classified in four distinct risk categories, with median overall survivals ranging between seven months to more than four years
[40][50].
4. Combined Treatments
Locoregional ablation therapies, such as radiofrequency ablation (RFA) and microwave ablation (MWA), are considered suitable alternatives to early stage HCC patients (BCLC 0 and A) who are not fit for surgery (resection or transplantation)
[41][51]. Using a needle electrode, RFA creates an electrical current in the radiofrequency range in order to provoke heat-based thermal cytotoxicity. By achieving a temperature range between 60–100 °C, this electrical current can cause instant thermocoagulation necrosis
[42][52]. RFA is most suitable for lesions up to 3 cm, with a margin of 0.5–1 cm of liver parenchyma needed in order to include any possible microscopic extension of the tumor
[43][53]. During ablation, energy disperses from the target lesion because of the cooling effect of hepatic blood flow. Due to this phenomenon, known as the heat-sink effect, RFA is less effective when the tumor is located near large vessels
[44][54]. MWA causes tissue necrosis by using high frequency electromagnetic energy. This energy leads a continuous rotation of dipole molecules, primarily water, in the microwave’s oscillating electric field, causing coagulation necrosis
[43][53]. MWA, when compared to RFA, can achieve higher temperature at the target lesion more rapidly and its efficacy is less influenced by heat-sink effect. This results in expansion of the ablation zone, allowing MWA to treat lesions up to 8 cm
[43][45][53,55].
5. Conclusions
The purpose of this review was to assess the indications, the optimal treatment schedule, the technical factors associated with TACEven t, and the overall application of TACE as a personalized treatment for HCC. Even though TACE is currently considered the first-line treatment for patients with HCC in the intermediate stage, recent studies have showed that it can beneficial for patients beyond stage B. Moreover, since BCLC stage B represents a heterogeneous group, not all intermediate-stage HCC patients benefit the same from TACE. Therefore, treatment allocation should be decided by a tumor board of specialists and each HCC patient should receive personalized treatment according to his/her individual features. Unfortunately, in many countries, a tumor board is not available in every hospital and physicians should address virtual boards remotely via telemedicine . Based on our experience and the review of the literature that we conducted, we propose a treatment algorithm regarding TACE procedure in HCC patients (Figure 2). In conclusion, TACE is an established procedure with proven efficacy and known adverse effects and contraindications. Nevertheless, additional studies and clinical trials are warranted to redefine patient selection criteria, introduce new indications, and stratify patients according to their individual prognostic evaluation.
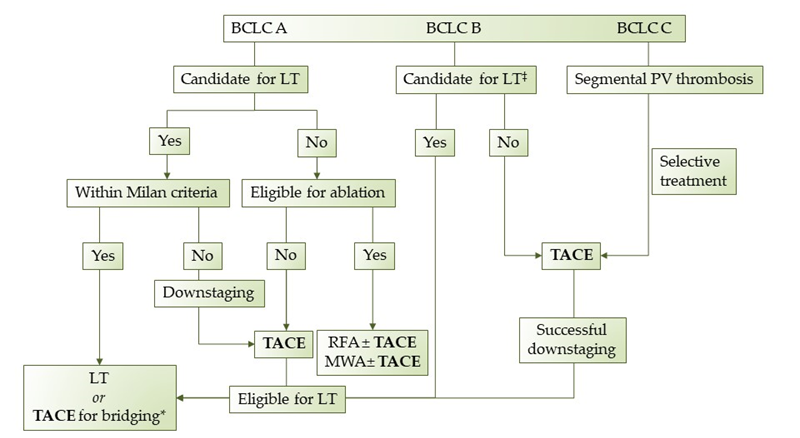
Figure 2. Proposed treatment algorithm regarding TACE in HCC patients. LT: Liver transplantation, TACE: transarterial chemoembolization, RFA: radiofrequency ablation, MWA: microwave ablation, *when in waiting list >6 months, ‡extended liver transplant criteria (size, AFP).