Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zihua Wang and Version 3 by Amina Yu.
Exosomes are naturally occurring nanoscale vesicles that are released and received by almost all cells in the body. Exosomes can be transferred between cells and contain various molecular constitutes closely related to their origin and function, including proteins, lipids, and RNAs. The importance of exosomes in cellular communication makes them important vectors for delivering a variety of drugs throughout the body.
- mesenchymal stem cell
- exosomes
- cardiovascular diseases
- neurodegenerative diseases
- drug delivery
1. Exosomes as a Therapeutic Tool
In the 1980s, extracellular vesicles (EVs), which can be secreted by almost all cells, were first discovered by P. Stahl [1][44] and R. Johnstone [2][45]. The biogenesis, secretion, and action mode of EV are illustrated in Figure 1. EVs are membrane-contained small vesicles, secreted by all types of prokaryote and eukaryote cells. The main category is apoptotic bodies, shedding micro vesicles and exosomes. In both physiological and pathological conditions, they can participates in cellular communication and convey specific information in origin and target cells; in physiological conditions, they can regulate homeostasis; or in pathological conditions, they can have bad effects, increasing tumorigenesis and metastasis, inflammation, and immune system activation [3][46]. Exosomes as a type of EV have Characteristics of the EVs and also have their own unique characteristics. They are membranous vesicles containing proteins, lipids, mRNAs, microRNAs, and other non-coding RNAs. Exosomes are cargos enclosed by a lipid bilayer membrane which contain cholesterol, phosphatidylserine, and sphingomyelin and ceramide and express a special subset of surface proteins such as membrane transport/fusion proteins, heat shock proteins (HSPs), and tetraspanins (CD9, CD63, CD81). In addition to electron microscopy used as a golden standard for identification of exosomes, the presence of CD9, CD63, and CD81 in exosomes helps facilitate their detection. Of note, exosomes may also express certain surface proteins, which define a very specific profile of exosomes [4][47]. According to body size, biological characteristics, and formation processes, EVs are mainly separated into exosomes, microvesicles, and apoptotic bodies [5][48]. At first, EVs were initially considered to be receptacles of cell waste biomaterial with no particular function. Later, researchers found that the EVs can achieve their biological activity by paracrine action of cells. Hence, increasing articles have emerged about the separation, identification, and function of EVs. Different methods have been used to isolate EVs from cells, including ultracentrifugation [6][7][49,50], immunological separation [8][51], ultrafiltration [9][52], size exclusion chromatography [9][52], polymer-based precipitation separation [10][53], magnetic separation [11][54], acoustic fluid separation, acoustic fluid separation [12][55], deterministic lateral displacement separation [13][56]. During the isolation technique, the most commonly used is differential centrifugation.
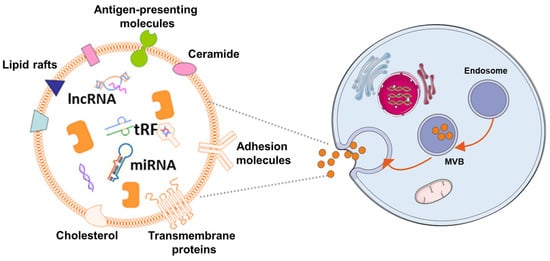
Figure 1. The mechanism of EV biogenes. Exosomes are released by cells of hematopoietic and non-hematopoietic origin. The EV production process can be summarized as follows: (1) the cytoplasmic membrane inward budding forms intracellular vesicles, which are termed as endosomes; (2) intracellular vesicles further develop to form multivesicular bodies (MVBs); (3) MVBs fuse with the cytoplasmic membrane to release exosomes, which can be incorporated into recipient cells through pinocytosis/phagocytosis or influence recipient cell signaling via ligand-receptor interaction.
Exosomes, the prominent EVs, typically have a cup-shaped structure under the electron microscope with a size of 30–150 nm. Exosomes are smaller than other EVs, and their size distribution is more uniform. As large and aggregated vesicles (>200 nm) can be trapped in the sinusoids or can be swallowed by macrophages, after systemic administration, smaller vesicles, like exosomes, can cross the endothelial barrier [14][57]. Exosomes are derived from their originating cell with bilayer membranes [15][58]. The membrane component of exosomes has a higher cholesterol content [16][59] and has a lower phosphatidylcholine level than donor-cell membranes [17][60]. The characters make them less susceptible to the permeation of small solutes and are more stable, and allow them to fuse with the receptor cells and release their contents inside [18][61]. Aside from their lipid composition, the surfaces of exosomes contain proteins and sugars, which makes exosomes change charge and maintenance of membrane structure, and can be the biomarker for exosome identification [19][62]. The contents of exosomes are lipids, proteins, and nucleic acids, such as microRNAs, and so onetc. [20][21][63,64], which play an essential role in intercellular communication and shows the possibility for using exosomes as a tool to treat disease or as biomarkers for early diseases diagnosis [22][36]. Although natural MSCs-Exo showed no significant difference in efficacy compared with MSCs transplantation, MSCs-Exo is more feasible for clinical application in the treatment of central nervous system injury due to its higher safety and stronger plasticity compared with MSCs. Currently, numerous studit hases have shown that exosomes are therapeutic in a variety of conditions, including neurodegenerative diseases [23][65], cardiovascular [24][66], and cerebrovascular diseases [25][67], and so on [26][68]. MSCs-derived exosomes (MSCs-Exo) not only have the characteristics of MSCs but the advantages of EVs. In contrast with MSCs, they use MSCs-Exo as cell-free therapeutics that offer several benefits, such as high stability, accessible storage, and low immunogenicity [27][28][29][30][69,70,71,72].
2. Exosomes Therapy for Cardiovascular Disease
It has been confirmed that MSCs transplantation helps recover the injured cardiomyocytes and preserves heart function in vivo and in vitro [31][32][33][34][35][15,18,82,83,84]. Focusing on these features, MSCs-Exo is also under-researched in many basic medical or preclinical studies [36][85]. In 2010, the first study of MSCs-Exo was firstly used for myocardial ischemia/reperfusion injury. Researchers proposed that MSCs-Exo contributes to myocardial repair [37][86]; however, the underlying mechanism is not entirely clear. Soon after that, several studies have illustrated the similar efficacy of MSCs-Exo for cardiovascular disease (CVD) treatment, and researchers tend to seek out the potential mechanism. Zhao and his colleagues found that MSCs-Exo attenuates myocardial ischemia/reperfusion injury via modifying the polarization of M1 macrophages to M2 macrophages. The possible signal system may be miR-182, a potent candidate mediator of macrophage polarization, and Toll-like receptor 4 (TLR4) as a downstream target [38][73]. Zhu et al. used hypoxia-conditioned MSCs-Exo (Hypo-MSCs-Exo) to administer to mice with a permanent condition of MI and found that the content of Hypo-MSCs-Exo facilitates ischemic cardiac repair by ameliorating cardiomyocyte apoptosis [39][74]. Another study by Lai et al. [37][86] showed that the infarct size was remarkably reduced by administrating MSCs-Exo in mice models of myocardial ischemia/reperfusion injury. Huang and his colleagues administered bmMSCs-derived exosomes (bmMSCs-Exo) to a rat model of acute MI (AMI) and demonstrated that MSCs-Exo could reduce scar size and restore heart function [40][75]. Sun et al. have shown that exosomes derived from using lentivirus containing HIF-1α overexpressing vector infected MSCs can rescue the impaired cardiac tissue by promoting neovessel formation and inhibiting fibrosis [41][76]. Bian et al. [42][77] administered MSCs-EVs to a rat model of AMI, reduced the scar size and preserved the diastolic and systolic function. Hirai and his group found that intracoronary injection of cardiosphere-derived exosomal microRNAs is safe and improves cardiac function in a swine model of dilated cardiomyopathy (DCM) [43][78]. In a study, Yu and his group found that this protective effect of MSCs-Exo was partly due to the content of exosomes, such as miRNA [44][79].
In recent years, many it hasstudies have also been attempting to identify various diseases by the early prognosis value of MSCs-Exo. It has beenRecent studies have suggested that plasma levels of exosome-related miRNAs could serve as new biomarkers in disease diagnosis [45][87]. These findings suggest that exosomes may also have some value in disease diagnosis.
3. Exosomes Therapy in Central Nervous System Disease
Neurodegenerative diseases are chronic, latent, progressive disorders that occur in the central nervous system (CNS). They are characterized by the loss of neuronal structure and function [46][88] lead to Information transmission disorder for neuron-to-neuron. Although great progress has been made in neuroscience research, there are still huge shortcomings. The main and most important is the lack of thorough understanding of its specific pathological mechanism. Moreover, the diseases of the nervous system are more complicated and multifactorial, which makes it more difficult to [47]study [6]. Due to the extremely weak self-regeneration of neurons, the dysfunction after central nervous system injury often accompanies patients for life, so it has become a difficult problem to be broken through in clinical treatment. In the treatment of central nervous system injury, MSCs-Exo has been proved to have definite therapeutic effects of promoting neurovascular regeneration, regulating inflammatory environment and repairing nerve myelin sheath. Many researchers have confirmed that stem-cell-derived exosomes have neuroprotection and neurotrophy effects [22][48][36,89] (Figure 2). Furthermore, the small size of MSCs-Exo makes itself cross the blood–brain barrier, reaching the brain or spinal cord tissue [22][36].
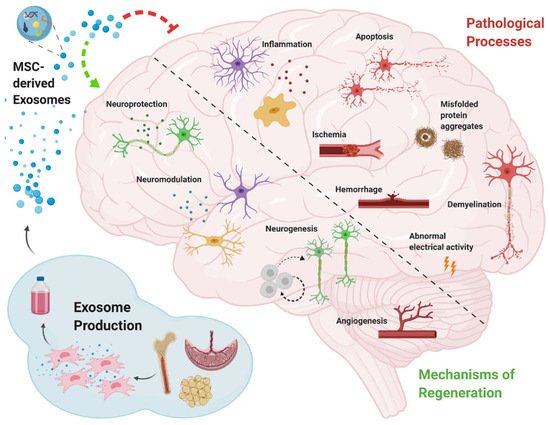
Stroke is one of the leading causes of death [49][96]. Some cell-based therapies have been deliberated to promote stroke treatment in preclinical and clinical trials [50][97], and the secretion of stem cells, such as exosomes, has also shown to be promising in various preclinical models of stroke recovery [51][98]. Xin et al. found that in rat models of stoke, administration of MSCs-Exo has been shown to enhance neurogenesis and angiogenesis and improve brain function recovery [52][95]. In amyotrophic lateral sclerosis (ALS), related studies have found that misfolded SOD1 protein can be transferred from cell to cell through exosomes dependence and exosomes independence, make progating diaeases [53][99]. In addition, ianot washer study indicated that TDP-43 was also associated with exosomes. Exosomes are an important pathway for THE transfer of TDP-43 aggregates [54][100]. Both SOD1 protein and TDP-43 protein are important pathological features of ALS. Exosomes also act through anti-apoptosis and anti-necrosis mechanisms (activating cell survival PI3K-B-cell lymphoma-2 (Bcl-2) pathway). Additionally, endogenous neuronal survival factors play an important role in the treatment of ALS by enhancing the receptor cells [55][101]. Riazifar and their colleagues assessed the effect of MSCs-Exo in treating multiple sclerosis and found that intravenous administration of MSCs-Exo can decrease neuroinflammation and reduce demyelination [23][65]. Additionally, in multiple sclerosis it often presents as chronic inflammation. MSCs-exo can also play an important role in this regard. It can regulate the activation of microglia by and by inhibiting the release of pro-inflammatory cytokines, greatly reducing the amount in the plasma to reduce inflammatory infiltration. A study [102] used [56]IFNγ-stimulated dendritic cell cultures were used to release exosomes that increased myelin levels and reduced oxidative stress and promote myelin reformation after demyelination. Chen and their partners administrated human adipose mesenchymal stem cell (HaMSC)-derived exosomes (HaMSC-Exo) into a weight-drop-induced traumatic brain injury (TBI) rat model and found that HaMSC-Exo promoted functional recovery, suppressed neuroinflammation, reduced neuronal apoptosis, and increased neurogenesis in TBI rats [57][80]. TIn a study to test the influence of MSCs-Exo in a large animal model of TBI, experts used MSCs-Exo to administrate female Yorkshire swine after TBI. They found that exosome-treated animals had significantly attenuated brain swelling and smaller lesion size, decreased blood-based cerebral biomarkers levels, and improved blood–brain barrier (BBB) integrity [58][81]. The inflammatory response activated after nerve injury may cause a secondary attack on the lesion. However, MSCs-Exo significantly prevented the pro-inflammatory cytokine release while promoting an M1 to M2 phenotype polarization in microglia and thereby reducing inflammatory damage. de Godoy, Mariana A. et al. have shown that MSC-EVS can reduce the expression of ROS related fluorescence signal in AD hippocampus neurons in vitro and protect neurons from Aβ protein-induced oxidative damage [59][103]. Recent studies have found that MSCs-Exo can significantly reduce the accumulation of β-amyloid (Aβ) protein in neurons, which confirming the therapeutic effect of MSC-EVS on the alleviation of pathological changes in Alzheimer’s disease [60][104]. In AD mice model, experts found that intracerebroventricularly injected BM-MSCs can improve cognitive impairment by ameliorating astrocytic inflammation as well as synaptogenesis [61][93]. In another study, MSCs-Exo was injected into APP/PS1 mice, and after a period of treatment, the ability of spatial learning and memory was significantly improved. The symptoms associated with AD were significantly improved. It was confirmed that the activation of SphK/S1P signaling pathway could reduce Aβ deposition and promote the recovery of cognitive function in AD mice [62][105]. As neurodegenerative diseases are characterized by the intracellular or extracellular aggregation of misfolded proteins [63][106], some experts intend to find early content changing of exosomes as biomarkers for AD/PD diagnosis. Yang et al. found that the serum exosomes-derived microRNA, miR-135a, -193b, and -384, are potential biomarkers for early AD diagnosis. In addition, MSC-EVs also has a regulatory effect on the microglial immune activated by Aβ, which can improve the neuronal survival in AD brain. Research found that MSC-EVS could inhibit microglia polarization to pro-inflammatory M1 subtype and increase the number of anti-inflammatory M2 subtype microglia in AD transgenic mice, and upregulate the expression of anti-inflammatory progenitor TGF-β and IL-10 in brain tissues [64][107]. This immunomodulatory effect is also involved in the protective effect of MSC-EVs on AD neurons. Additionally, researchers discovered that the exosomes from AD patients might become toxicity vesicles containing toxic amyloid-beta protein [65][108]. That also illustrates that exosomes are closely related to the occurrence of central nervous system disorder/disease (CNSD)CNSD. Wang et al. development of exosome as a carrier for curcumin prevents neuronal death in vitro and in vivo to alleviate AD symptoms. ItThis study provides potential clinical evidence for exosome-based drug delivery in the treatment of AD [66][109]. Meckes Jr et al. found that 5 × FAD mice received hMSCs-Exo treatment can slow down AD pathogenesis and ameliorate inflammatory marker glial fibrillary acidic protein (GFAP) in a preclinical mouse model [67][92]. However, the efficacy of MSCs-Exo demonstrated only from the perspective of Aβ protein may require further validation in future clinical trials. Exosome-associated miR-137 has been found to be upregulated in neurons in PD, where it plays a vital role in neuronal oxidative stress induction. MiR-137 directly targets oxidation resistance-1 (OXR1) to negatively regulate its expression, thereby inducing oxidative stress. The levels of miRNAs have also been investigated in some PD models, such as in a manganese model where 12 miRNAs were significantly increased in exosomes; these miRNAs were shown to regulate key PD pathogenesis pathways including autophagy, inflammation and protein aggregation [68][110]. Another group reported that exosome delivery of hydrophobically modified siRNA to the brain efficiently targeted mHtt mRNA in a Huntington’s disease model, which is encouraging for the potential use of siRNAs to target α-syn in PD [69][111]. MSC-derived exosomes proved effective at rescuing dopaminergic neurons in the 6-OHDA mouse model of PD, and they can also carry miRNAs and interact with neuronal cells to reduce neuroinflammation and promote neurogenesis in mouse PD models [70][112]. In anot her study, also useding a 6-OHDA mouse MODEL of PD, treatment with SHED-derived exosomes was carried out. The expression level of TH in striatum and substantia nigra was decreased, demonstrating the potential of exosomes in PD treatment [71][113]. Whilst further investigations and clinical trials are required to confirm the benefits of therapeutic application of exosomes in PD, mounting evidence supports that the separation of exosomes from various cell types and their modification to target specific brain regions may hold therapeutic benefits for PD, among other disorders [72][114].
With the continuous development of MSCs-Exo research, a large number of research achievements have been made on the therapeutic effect and potential mechanism of MSCs-Exo in a variety of CVD and CNSD, showing great potential for disease treatment. However, at present, the treatment-related research of MSCs-Exo is still in the early stage, and there are many research gaps and problems to be explored and solved [73][74][115,116]. First, exosome bioactivity must be detected precisely. Only when exosomes have biological activity can they show function for treating or diagnosing various diseases. Second, exosomes need to be monitored in vivo for tracking biodistribution and targeting. Third, there need standardizations for exosomes composition, using dose, production, and so onetc. In addition, the question about how to modulate the bioactivity of exosomes is also required to be considered.
