Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Bruce Ren and Version 1 by Esubalew Kasaw Kasaw Gebeyehu.
The use of hydrogel in tissue engineering is not entirely new. In the last six decades, researchers have used hydrogel to develop artificial organs and tissue for the diagnosis of real-life problems and research purposes. Trial and error dominated the first forty years of tissue generation. Nowadays, biomaterials research is constantly progressing in the direction of new materials with expanded capabilities to better meet the current needs.
- conductive hydrogel
- cellulose
- tissue engineering
- hydrogel design and characterization
- electro-active tissues
1. Introduction
Every year, millions of people lose tissue or organs as a result of accidents or illnesses [1]. Tissue or organ transplantation is used to treat these patients. This approach, however, is constrained by the lack of donors. To solve the problem of the severe shortage of organ transplants, intensive research work, a review [2], has been performed in the last four decades to develop artificial organs and tissue for diagnosis and research purposes. Cells and their extracellular subassemblies are used to develop biological tissues for body repair, primarily with bio-based material scaffolds. The scaffolds support cellular attachment and regulate tissue shape. Some of the strategies used to develop scaffolds were tri-dimensional textiles [3[3][4],4], aerogel [5[5][6],6], hydrogels [1], nanofibers [7[7][8],8], and composites [9]. In all cases, scaffolds should ideally be sufficiently porous to enable the growth of cells, nutritional diffusion, and physiologic waste extraction [10]; have adequate tensile strength and elasticity [11]; have controlled degradation [12]; and possess suitable chemistry for cell adhesion [13]. Other desirable properties, such as electrical conductivity, in polymers have also been reported to accelerate the nerve regeneration in artificial nerve grafts [14]. However, the importance of electrically conductive hydrogels in tissue engineering has received insufficient attention as yet [15].
A hydrogel is a tri-dimensional polymeric material that can take the form of a matrix, film, liquid, or microsphere [16] which is water insoluble and has the ability to swell and preserve a significant amount of water, typically greater than the mass ratio of the polymer materials in their interstitial structures [17]. Due to the presence of hydrophilic groups, such as –NH2, –OH, –COOH, and –SO3H, in their polymer networks and osmotic pressure, hydrogels continue to absorb and swell upon contact with water to form 3D structures. Physical or chemical crosslinking causes the ability of hydrogels to maintain an unaltered 3D structure during swelling, and this also helps to prevent hydrogels from dissolving in the solvent [18]. Upon hydration in an aqueous environment, hydrophilic groups or domains of polymeric networks form the hydrogel structure presented in Figure 1 [19].
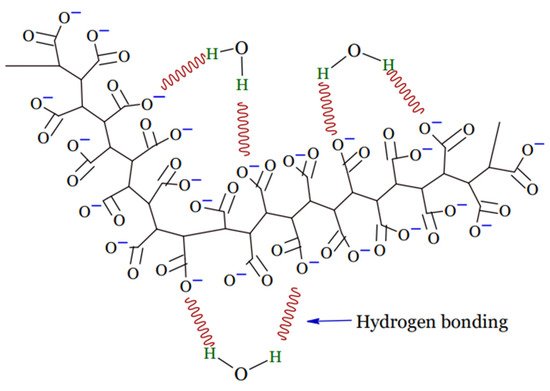
Figure 1.
Structure of hydrogel.
Hydrogel products are classified based on a variety of criteria, as shown in Figure 2. The origins of the polymeric constituent of hydrogel can be classified as synthetic [20], hybrid [21], and natural [22]. Hydrogels from natural sources [22] can be derived from polysaccharide-based materials, such as cellulose [23]; chitosan [24]; glycosaminoglycans [25]; alginate [26,27][26][27]; protein-based materials, such as, silk fibroin [28[28][29],29], collagen [30], elastin [31], gelatin [32], and fibrin [33]; and decellularized hydrogels [34,35][34][35]. The integrity of the hydrogel is maintained through chemical crosslinking, physical crosslinking, or both [17,19,36,37,38][17][19][36][37][38]. Physical crosslinking, such as heating/cooling, hydrophobic interactions, freezing–thawing, complex coacervation, ionic interactions, and hydrogen bonding, have resulted in temporary networks of hydrogels, whereas permanent junctions exist in chemically crosslinked networks [16] obtained by employing grafting, chemical crosslinkers, radiation crosslinking, enzymatic reactions, click chemistry, radical polymerization, and thermo-gelation. A crosslinked network is a set of one, two, or three, and maybe more, types of monomers referred to as homopolymer, copolymer, and multipolymer, respectively [17]. Perhaps the hydrogel network is arranged in a network to swell up in a monomer; afterward, it reacts by forming a second intermeshing network structure to form interpenetrating polymeric composition [20].
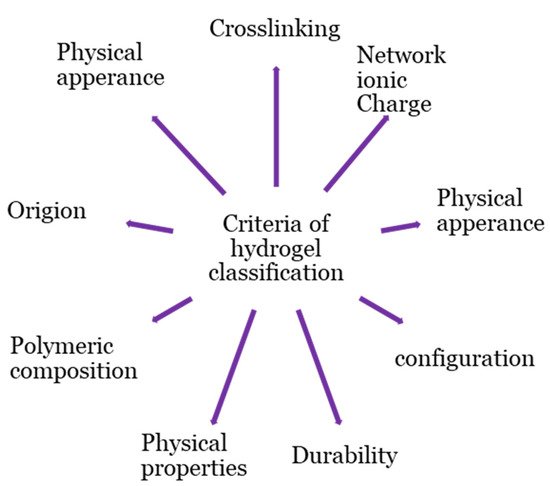
Figure 2.
Classification bases of hydrogel.
The spatiotemporal control of hydrogel physicochemical characteristics is essential for monitoring their dynamics, such as durability and orientation [39]. The orientational dynamic appearance of biomolecules may be either crystalline or amorphous.
Hydrogels can be biodegradable or non-biodegradable in terms of durability [20]. Hydrogel physical properties have been advanced from conventional to smart [16]. Smart hydrogels are stimuli-sensitive and change the volume of the system structure in response to various stimuli, such as electric, light, temperature, and pH [17,40,41,42][17][40][41][42].
ThHe work presented in this review re aims to demonstrate the possibilities for producing conductive hydrogel of cellulosic-based materials. The organic compound cellulose was preferred for the hydrogel base material in this review, due to its excellent inherent properties, such as renewability [43[43][44],44], absorption ability [45], hygroscopicity [46], air permeability [47], biocompatibility [48,49[48][49][50],50], stability [51], bioactivity [50], and biodegradability [52]. In contrast to some of the other studies discussed in this review, however, design parameters’ consideration and characterization of hydrogel scaffolds for electro-active tissues, in general, and the preparation techniques of cellulosic-based conductive hydrogel, in particular, are given a place of due attention on this list.
2. Hydrogel Conductivity Inclusion
2.1. Electro-Active Tissues
Electrical conductivity is an integral component of the human body [83][53]. Neurons function as a result of interacting networks woven by nerve cells. The nervous system is thought to contain approximately a trillion neurons. These highly irregularly shaped cells have the basic properties of the nervous system, such as intrinsic electrical conductivity. The ability of neurons to transmit signals from one neuron to another, as well as from a neuron to muscles and glands, is referred to as conductivity. The cell membrane allows a relatively large amounts of potassium ions to diffuse out of the cell, while allowing only a small amount of sodium ions to enter. These diffusive movements are simply the result of these ions moving down concentration gradients, following active transport by the sodium–potassium pump. When a voltage-gated ion channel opens, positively charged sodium ions diffuse into the axon, changing the membrane potential from −70 mv to zero and even higher, frequently reaching +35 mv. The membrane is said to have depolarized at that point. It happens in about a half-millisecond. The sodium gate then closes, and the usual outward diffusion of potassium occurs, causing the membrane potential to return to −70 and possibly lower to −73, due to a temporary overshoot in outward diffusion of potassium. This return to resisting is referred to as repolarization. Repolarization takes approximately half a millisecond. Thus, an action potential is a depolarization that is followed by a repolarization that takes about a millisecond to complete for a set of cells and tissues to function [55,84][54][55].
Electrical stimulation is a concern that is specific to a subset of cell types, including neurons and myocytes, in nerve-tissue engineering [85][56] (Table 1). As a result, electro-active biomaterials are required [61][57]. To meet these performance requirements, cellulose scaffolds coated with conductive materials can be used. Such materials have defined pore sizes, physicochemical characteristics, and electrical conductivities; they are also biocompatible and promote neurological differentiation [57][58].
2.2. Electro-Active Hydrogel
Cellulose scaffolds are an excellent material for nerve neurogenesis, due to their customizable surface chemistry and mechanical characteristics. Perhaps, to improve integrin-based attachment and cell–scaffold interactions, cellulose materials can be chemically modified and protein-coated [57][58]. Electro-active biomaterial-mediated stem-cell differentiation into specific cell lineages is of great significance for tissue regeneration. Although the underlying molecular events and mechanism of electro activation are not fully understood, there are some general guidelines for designing conductive hydrogels. Aside from matching the morphology and mechanical properties of hydrogels to the tissue microenvironment, it is critical to mimic the tissue electrophysiological environment. Neurons form synapses to transmit electrical signals and integrate into neuronal circuits in the mature nervous system. Neurons switch from an active electrical transmission state to an electrically silent and growth-competent state after axonal injury. When a cell shifts a single cell to multicellular collections and tissues, a striking parallel is found. Cells are regulated not only by their own potential, but also the potential of their neighboring cells via gap junctions [84,86][55][59].
Electrically conductive materials and crosslinked hydrogel networks are used to create conductive hydrogels through co-networks, blends, and self-assembly. This can be achieved through post-polymerization of a conducting monomer in a prefabricated hydrogel; composite strategies involving the mixing of conductive materials/monomers and hydrophilic polymers/monomers, followed by simultaneous or stepwise crosslinking to produce conductive hydrogels; and in situ polymerization involving the self-assembly of the modified electrically conductive materials [87][60]. The pros and cons of the strategies are given in Table 2.
Table 2. Advantages and disadvantages of different design strategies for preparing conductive hydrogels (copied from Reference [87][60]).
| Design Strategies | Advantages | Disadvantages | ||||
|---|---|---|---|---|---|---|
| In situ polymerization |
|
|
||||
| Post-polymerization |
|
|
||||
| Grey matter | ||||||
| 0.10 | Stomach | 0.50 | ||||
| Hypothalamus | 0.08 | Stomach contents | 0.35 | |||
| Eye lens | 0.25 | Tendon | 0.30 | |||
| Composite strategies |
|
| Pineal body | 0.08 | Testis | 0.35 |
| Pituitary | 0.08 | Thyroid gland | 0.50 | |||
|
|
Salivary gland | 0.35 | Trachea | 0.35 | |
| Thalamus | 0.08 | Urine | 0.70 | |||
| Tongue | 0.30 | Blood | 0.70 | |||
| White matter | 0.06 | Cortical bone | 0.02 | |||
| Adrenals | 0.35 | Bone marrow | 0.06 | |||
| Bladder | 0.20 | Cartilage | 0.18 | |||
| Large intestine | 0.10 | Fat | 0.04 | |||
| Duodenum | 0.50 | Muscle | 0.35 | |||
| Esophagus | 0.50 | Nerve (Spinal cord) | 0.03 | |||
| Bile | 1.40 | Skin | 0.10 | |||
| Gall bladder | 0.20 | Tooth | 0.02 | |||
| Heart | 0.10 | Ligament | 0.30 |
Different types of conductive materials exhibit varying properties. Research (a review by Rong et al., 2018) [88][61] shows that three classes of materials are used on hydrogels for conductive purposes: metals, carbon allotropes, and intrinsically conductive polymers (ICPs).
For semi-conductor hydrogels, ionomers and silicones may be used as conducting materials as well [88][61]. As just an instance, when cellulose dissolved in an aqueous solution of benzyltrimethyl ammonium hydroxide (BzMe3NOH), ionic conductive cellulose hydrogels (CCHs) with anti-freezing properties were directly fabricated by chemical crosslinking without further treatment [89][62].
3. Incorporation of Conductive Materials on to Cellulose Hydrogels
3.1. Intrinsically Conductive Polymers (ICPs)
ICPs are conjugated polymers that have an extended delocalized system of π electrons that generally runs along the polymer backbone and is made conductive through doping [90][63]. The free motion of the loosely held π electrons within the unsaturated segments can open an electrical path for itinerant charge carriers. However, the changes in surface zeta potential and polymer surface properties, such as wettability and spatial conformation, can affect the cell behavior of electrical stimulation behavior [91][64]. It was subsequently understood that several polymers, such as polyacetylene, polypyrrole (PPy), polyaniline, polythiophene, poly (p-phenylene), and poly (3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT/PSS), are conjugated polymers whose electrical conductivity is dramatically increased by doping. Doping involves the addition of a small amount of a chemical agent, which alters the electronic structure. The doping process, on the other hand, is reversible and involves a redox process.
Two major fabrication routes have already been investigated for the development of conductive polymer hydrogels by using ICP: gelation of CPs and hydrophilic polymers/monomers by self-assembly or the introduction of cross-linkable elements, as well as chemical oxidation; and electrochemical polymerization in a prefabricated hydrogel [88][61]. In a specific instance, X. Liang et al. developed a conductive hydrogel by polymerizing PPy through a prefabricated of chemically crosslinked microcrystalline cellulose (MMC) [73][65]. Gelation and chemical physical polymerization were employed after mixing the bacterial cellulose (BC) and PEDOT/PSS [92][66] to enhance the conductivity also.
3.2. Carbon Allotropes
Carbon-based materials are regarded as promising conductive materials for the fabrication of conductive hydrogels, due to their unique properties of high electrical conductivities, excellent environmental stability, and low production costs [88][61]. Materials that consist of only carbon atoms can have a wide range of conductivities, from the insulator diamond to conductors such as charcoal [93][67], carbon black (CB), graphene, and carbon nanotubes (CNTs) [94,95][68][69]. The level of conductivity will depend on the degree of delocalized electrons, thus making the graphitization and purity of the carbon compounds important factors [96][70]. Carbon-based biomaterials are commonly used as reinforcing agents in tissue-engineering applications to improve the mechanical performance and conductivity of the polymer matrix. Along with their unique mechanical properties, chemical stability, large specific surface area, and high electrical conductivity, graphene and carbon nanotube-based materials are the most widely used in tissue engineering. Furthermore, their large surface area and abundance of functional groups aid in the loading and release of bioactive species, such as chemical drugs, growth factors, genes, and proteins [83][53].
Blending with various polymers and self-assembly after modification are the two most common ways of preparing carbon-based conductive hydrogels [88][61]. Cellulose nanocrystals were grafted in to phenylboronic acid (CNCs-ABA) and multi-walled carbon nanotubes (MWCNTs) to develop electrical conductivity [97][71]. Another illustration is the post-polymerization of MWCNTs, with graphene powder (r-GOx) to adhere to pure regenerated cellulose-based electrolyte membranes [79][72].
Sometimes more than one conductive material may be used to enhance the conductivity of hydrogel. In a specific instance, to develop a conductive hydrogel, bacterial cellulose (rBC) slurry was mixed with PPy and single-walled carbon nanotubes (SWCNTs) and crosslinked in a stepwise manner. After preparing the rBC/PPy hydrogel, CNTs were added to the prepared rBC/PPy solution and dispersed before physical crosslinking had occurred [75][73]. The different preparation techniques of cellulosic-based conductive hydrogels is illustrated in Table 3.
3.3. Metals
Metals’ exceptional features, such as high conductivity, optical, magnetic, and catalytic properties, as well as metallic nanoparticles/nanowires, such as Al, Au, Ag, Cu, etc, have been widely used in the fabrication of conductive hydrogels [98][74]. Due to their high mechanical properties, fatigue resistance, and conductivity, bulk metals, such as titanium, magnesium, and stainless steel, have been used as bone-repair implants [83][53]. Although metals have some drawbacks, such as lack of flexibility, toxicity, cost, and negative environmental effects, they remain the only viable alternative for applications requiring high conductivity [91,99][64][75].
The common methods to develop metal-based conductive hydrogels are UV crosslinking and the in situ polymerization of hydrogel monomer and reduction of metal ions, using reducing agent [88][61]. An illustration of in situ polymerization through simultaneous crosslinking was performed by blending a precursor cellulose microcrystalline (CMC) solution; a monomer acrylic acid, initiator ammonium persulfate, catalyst N,N,N′,N′ tetramethylethylenediamine and crosslinker aluminum hexahydrate (AlCl3.6H2O), and the conductive materials of metallic ions of Al3+ produced conductive hydrogels [82][76]. Another example is grafting of acrylonitrile (AN) and acrylamide copolymers onto the hydroxypropyl methylcellulose (HPMC) chains in the presence of zinc chloride (ZnCl2), using ceric ammonium nitrate (AM) as the initiator [76][77]. In situ polymerization to form nanocomposite hydrogels of tannic acid–coated cellulose nanocrystal (TA@CNCs) ionic gel and then immersion in Al3+ solution to produce ionic coordination [100][78] have also been reported to develop cellulosic-based conductive hydrogel.
Table 3.
Preparation techniques of cellulosic-based conductive hydrogel.
| Hydrogel Features | Method of Crosslinking | Hydrogel Material | Conductivity (S/m) | (Potential) Application | Reference |
|---|---|---|---|---|---|
| Electro-active | Composite strategies | rBC/PPy and rBC/PPy/CNT | 6.2 × 10−2 | Cell proliferation | [75][73] |
| Conductive | Post-Polymerization | MCC/PPy | 0.783 | Electrochemical biosensors, electro-stimulated controlled drug release, and neural prosthetics | [73][65] |
| Conductive, self-healing, and strain- and thermal-sensitive performance | In situ polymerization | PAA-CMC-Al3+ | 162 | Flexible and wearable temperature-sensing devices | [82][76] |
| Self-healing, shape memory, and biocompatible | Composite strategies | CNCs-ABA | 3.8 × 10−2 | Strain sensors | [97][71] |
| Ultra-stretchable, tough, anti-freezing, and conductive | Composite strategies via graft polymerization | HPMC-g-P (AN-co-AM) | 1.54 | Strain Sensor | [76][77] |
| Transparent, anti-freezing, and ionic conductive | Chemical crosslinking | CCHs | 2.37 | Sensor | [89][62] |
| Thermally stable, crystalline, and electroactive | Composite strategies | Polyvinyl alcohol cellulose (PC) | Actuator | [74][79] | |
| Anisotropic and conductive, with high water content | Composite strategies | BC-PEDOT/ PSS | Scaffolds, implantable biosensors, and smart soft electronic devices | [92][66] | |
| Tough, stretchable, self-adhesive, self-healing, and strain-sensitive | In situ polymerization | TA@CNCs | Conductivity is proved by light emitting diode | Wearable electronic sensors and healthcare monitoring | [100][78] |
| Electroactive and ultrafast for electro-mechanical response | Post-polymerization | Cellulose-based all-hydrogel artificial muscles membrane. | 0.83–2.49 | Transportation of nerve impulses from human muscle | [79][72] |
References
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880.
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430.
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.; Guilak, F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839.
- Guilak, F.; Moutos, F. Three-Dimensional Fiber Scaffolds for Tissue Engineering. U.S. Patent 11/406,519, 25 February 2007.
- Wei, Z.; Wu, C.; Li, R.; Yu, D.; Ding, Q. Nanocellulose based hydrogel or aerogel scaffolds for tissue engineering. Cellulose 2021, 28, 7497–7520.
- Yahya, E.B.; Amirul, A.A.; Abdul Khalil, P.S.A.; Olaiya, N.G.; Iqbal, M.O.; Jummaat, F.; Atty Sofea, K.A.; Adnan, A.S. Insights into the Role of Biopolymer Aerogel Scaffolds in Tissue Engineering and Regenerative Medicine. Polymers 2021, 13, 1612.
- Jayaraman, K.; Kotaki, M.; Zhang, Y.; Mo, X.; Ramakrishna, S. Recent advances in polymer nanofibers. J. Nanosci. Nanotechnol. 2004, 4, 52–65.
- Mo, X.; Xu, C.; Kotaki, M.; Ramakrishna, S. Electrospun P (LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890.
- Burg, K.J. Tissue Engineering Composite. US Patent No. 6,991,652, 31 January 2006.
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Amsterdam, The Netherlands, 2000; pp. 175–189.
- Al-Munajjed, A.A.; Hien, M.; Kujat, R.; Gleeson, J.P.; Hammer, J. Influence of pore size on tensile strength, permeability and porosity of hyaluronan-collagen scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2859–2864.
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169.
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486.
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 164–174.
- Jiang, L.; Wang, Y.; Liu, Z.; Ma, C.; Yan, H.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; Sun, X. Three-Dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application. Tissue Eng. Part B Rev. 2019, 25, 398–411.
- Ebara, M.; Kotsuchibashi, Y.; Uto, K.; Aoyagi, T.; Kim, Y.-J.; Narain, R.; Idota, N.; Hoffman, J.M. Introductory Guide to Smart Biomaterials. In Smart Biomaterials; Springer: Tokyo, Japan, 2014; pp. 1–7.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174.
- Gulrez, S.K.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Application; Books on Demand: Norderstedt, Germany, 2011; pp. 117–150.
- Gibas, I.; Janik, H. Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol. 2010, 4, 297–304.
- Wang, C.; Stewart, R.J.; KopeČek, J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature 1999, 397, 417–420.
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115.
- Pal, D.; Nayak, A.K.; Saha, S. Cellulose-Based Hydrogels: Present and Future; Springer: Singapore, 2019; pp. 285–332.
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99.
- Atallah, P.; Schirmer, L.; Tsurkan, M.; Limasale, Y.D.P.; Zimmermann, R.; Werner, C.; Freudenberg, U. In situ-forming, cell-instructive hydrogels based on glycosaminoglycans with varied sulfation patterns. Biomaterials 2018, 181, 227–239.
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230.
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633.
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792.
- Wang, H.Y.; Zhang, Y.Q. Processing silk hydrogel and its applications in biomedical materials. Biotechnol. Prog. 2015, 31, 630–640.
- Griffanti, G.; Jiang, W.; Nazhat, S.N. Bioinspired mineralization of a functionalized injectable dense collagen hydrogel through silk sericin incorporation. Biomater. Sci. 2019, 7, 1064–1077.
- Wright, E.R.; McMillan, R.A.; Cooper, A.; Apkarian, R.P.; Conticello, V.P. Self-Assembly of Hydrogels from Elastin-Mimetic Block Copolymers. MRS Online Proc. Libr. 2002, 724.
- Yamamoto, M.; Ikada, Y.; Tabata, Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J. Biomater. Sci. Polym. Ed. 2001, 12, 77–88.
- Ye, K.Y.; Sullivan, K.E.; Black, L.D. Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. J. Vis. Exp. JoVE 2011, 55, e3251.
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038.
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658.
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone Tissue Engineering: A Review. Nanomaterials 2020, 10, 1511.
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci. 2014, 4, 25–31.
- Esa, F.; Tasirin, S.M.; Abd Rahman, N. Overview of bacterial cellulose production and application. Agric. Agric. Sci. Procedia 2014, 2, 113–119.
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372.
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv. Healthc. Mater. 2013, 2, 218–227.
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301.
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627.
- De Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira Junior, O.B.; Ribeiro, S.J. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420.
- Bhat, A.; Khan, I.; Usmani, M.A.; Umapathi, R.; Al-Kindy, S.M. Cellulose an ageless renewable green nanomaterial for medical applications: An overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 2019, 129, 750–777.
- Bashari, A.; Rouhani Shirvan, A.; Shakeri, M. Cellulose-based hydrogels for personal care products. Polym. Adv. Technol. 2018, 29, 2853–2867.
- Nandakumar, D.K.; Ravi, S.K.; Zhang, Y.; Guo, N.; Zhang, C.; Tan, S.C. A super hygroscopic hydrogel for harnessing ambient humidity for energy conservation and harvesting. Energy Environ. Sci. 2018, 11, 2179–2187.
- Filipova, I.; Irbe, I.; Spade, M.; Skute, M.; Dāboliņa, I.; Baltiņa, I.; Vecbiskena, L. Mechanical and air permeability performance of novel biobased materials from fungal hyphae and cellulose fibers. Materials 2021, 14, 136.
- Fricain, J.; Granja, P.; Barbosa, M.; De Jéso, B.; Barthe, N.; Baquey, C. Cellulose phosphates as biomaterials. In vivo biocompatibility studies. Biomaterials 2002, 23, 971–980.
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878.
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788.
- Teeri, T.T.; Brumer, H., III; Daniel, G.; Gatenholm, P. Biomimetic engineering of cellulose-based materials. Trends Biotechnol. 2007, 25, 299–306.
- Torgbo, S.; Sukyai, P. Biodegradation and thermal stability of bacterial cellulose as biomaterial: The relevance in biomedical applications. Polym. Degrad. Stab. 2020, 179, 109232.
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, e2007429.
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179.
- Llinas, R.R. Intrinsic electrical properties of mammalian neurons and CNS function: A historical perspective. Front. Cell. Neurosci. 2014, 8, 320.
- Hirata, A.; Takano, Y.; Kamimura, Y.; Fujiwara, O. Effect of the averaging volume and algorithm on the in situ electric field for uniform electric- and magnetic-field exposures. Phys. Med. Biol. 2010, 55, N243–N252.
- Hu, X.; Ricci, S.; Naranjo, S.; Hill, Z.; Gawason, P. Protein and Polysaccharide-Based Electroactive and Conductive Materials for Biomedical Applications. Molecules 2021, 26, 4499.
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45.
- Hardy, J.G.; Cornelison, R.C.; Sukhavasi, R.C.; Saballos, R.J.; Vu, P.; Kaplan, D.L.; Schmidt, C.E. Electroactive Tissue Scaffolds with Aligned Pores as Instructive Platforms for Biomimetic Tissue Engineering. Bioengineering 2015, 2, 15–34.
- Xu, J.; Tsai, Y.L.; Hsu, S.H. Design Strategies of Conductive Hydrogel for Biomedical Applications. Molecules 2020, 25, 5296.
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chemistry 2018, 24, 16930–16943.
- Wang, Y.; Zhang, L.; Lu, A. Transparent, Antifreezing, Ionic Conductive Cellulose Hydrogel with Stable Sensitivity at Subzero Temperature. ACS Appl. Mater. Interfaces 2019, 11, 41710–41716.
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymers 2019, 11, 350.
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448.
- Liang, X.; Qu, B.; Li, J.; Xiao, H.; He, B.; Qian, L. Preparation of cellulose-based conductive hydrogels with ionic liquid. React. Funct. Polym. 2015, 86, 1–6.
- Qian, C.; Higashigaki, T.; Asoh, T.A.; Uyama, H. Anisotropic Conductive Hydrogels with High Water Content. ACS Appl. Mater. Interfaces 2020, 12, 27518–27525.
- Kasaw, E.; Haile, A.; Getnet, M. Conductive Coatings of Cotton Fabric Consisting of Carbonized Charcoal for E-Textile. Coatings 2020, 10, 579.
- Hu, X.; Tian, M.; Qu, L.; Zhu, S.; Han, G. Multifunctional cotton fabrics with graphene/polyurethane coatings with far-infrared emission, electrical conductivity, and ultraviolet-blocking properties. Carbon 2015, 95, 625–633.
- Rahman, M.J.; Mieno, T. Conductive Cotton Textile from Safely Functionalized Carbon Nanotubes. J. Nanomater. 2015, 2015, 1–10.
- Coeuret, F. Electrical Conductivity of Carbon or Graphite Felts. Available online: https://www.electrochem.org/dl/ma/203/pdfs/2277 (accessed on 29 January 2022).
- Xiao, G.; Wang, Y.; Zhang, H.; Zhu, Z.; Fu, S. Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int. J. Biol. Macromol. 2021, 170, 272–283.
- Sun, Z.; Yang, L.; Zhao, J.; Song, W. Natural Cellulose-Full-Hydrogels Bioinspired Electroactive Artificial Muscles: Highly Conductive Ionic Transportation Channels and Ultrafast Electromechanical Response. J. Electrochem. Soc. 2020, 167, 047515.
- Wang, L.; Hu, S.; Ullah, M.W.; Li, X.; Shi, Z.; Yang, G. Enhanced cell proliferation by electrical stimulation based on electroactive regenerated bacterial cellulose hydrogels. Carbohydr. Polym. 2020, 249, 116829.
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865.
- Holmström, E.; Lizárraga, R.; Linder, D.; Salmasi, A.; Wang, W.; Kaplan, B.; Mao, H.; Larsson, H.; Vitos, L. High entropy alloys: Substituting for cobalt in cutting edge technology. Appl. Mater. Today 2018, 12, 322–329.
- Pang, J.; Wang, L.; Xu, Y.; Wu, M.; Wang, M.; Liu, Y.; Yu, S.; Li, L. Skin-inspired cellulose conductive hydrogels with integrated self-healing, strain, and thermal sensitive performance. Carbohydr. Polym. 2020, 240, 116360.
- Chen, D.; Zhao, X.; Wei, X.; Zhang, J.; Wang, D.; Lu, H.; Jia, P. Ultrastretchable, Tough, Antifreezing, and Conductive Cellulose Hydrogel for Wearable Strain Sensor. ACS Appl. Mater. Interfaces 2020, 12, 53247–53256.
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121.
- Jayaramudu, T.; Ko, H.-U.; Zhai, L.; Li, Y.; Kim, J. Preparation and characterization of hydrogels from polyvinyl alcohol and cellulose and their electroactive behavior. Soft Mater. 2016, 15, 64–72.
More
