Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 6 by Jessie Wu and Version 8 by Jessie Wu.
Cannabis sativa L. is known for its medicinal uses since ancient times, because of its rich supply of phytochemicals, hence the quest for harnessing its pharmacological potential by scientists. The term “Cannabis” is used to define the products (drugs and essential oils) that are prepared or obtained from the annual herb C. sativa and its variants, which are of the family Cannabaceae. C. sativa has been used for the treatment of rheumatism, epilepsy, asthma, skin burns, pain, the management of sexually transmitted diseases, difficulties during child labor, postpartum hemorrhage, and gastrointestinal activity.
- medicinal plant
- Cannabis sativa
- phytochemicals
- bioactivity
- extraction methods
- characterization
1. Origin and Botanical Description of C. sativa
The genus name Cannabis means “cane-like” while sativa means “sown”, which signifies that the plant is propagated from the seed and not from the roots [1]. It is believed to have originated in Asia and occurs widely in Africa [1][2]. Central and south-east Asia are the potential natural origins for the domestication of the Cannabis genus [3] and it is known by different common names in different languages (hemp, marihuana, kannabis sativa, ganja, bhang, and al-bhango) [4]. In South Africa, it is colloquially known, in Afrikaans, as “dagga”; in IsiXhoxa as “umfincafincane”; and in Isizulu as “umunyane” [5][6]. Taxonomically, Carl Linnaeus, a Swedish botanist, was the first to coin the name Cannabis sativa [7]. Other botanists stated that different types of Cannabis existed based on their size, shape, and resin content (breeding and selection).
The Cannabis phenotype (its observable traits or characteristics, such as its leaf shape and flower color) is based on two main factors: its genetic code (genotype) and the external environmental factors [8].
The roots are branched and are about 30–60 cm deep (Farag and Kayser, 2017) [1]. Cannabis inflorescence is made up of several flower heads found on long leafy stems from each leaf axil. A single brownish fruit, about 2-5mm long, is produced per flower, and it contains a single seed tightly covered with a hard shell [1]. The fruit is propagated by bird and the seed germinates after 8–12 days [7]. The leaves, bracts, and stems of the plant are rich in trichrome, which are a diverse set of structures containing the secondary metabolites (phytocannabinoids and terpenoids) responsible for the defense, plant interactions, and typical smell [7]. Figure 1, below, shows the plant parts of C. sativa.
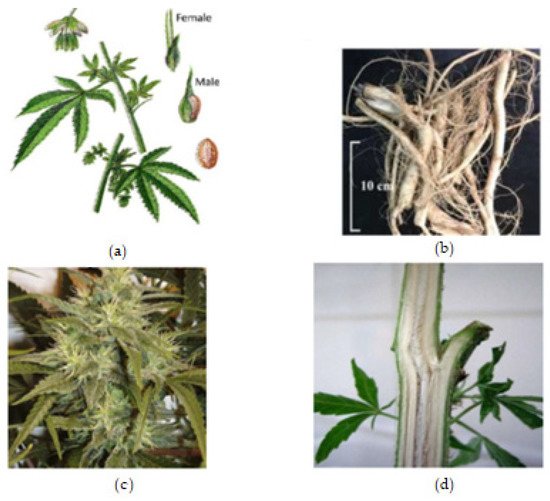
Figure 1. Cannabis plant parts. (a) Male and female Cannabis flowering parts with fresh leaf and seed. (b) Fresh Cannabis root. (c) Fresh Cannabis inflorescence (flower). (d) Fresh Cannabis stem bark.
2. Phytochemistry of C. sativa
2.1. Chemical Profile of C. sativa
Cannabis, as a herbal medicine, is a complex mixture of compounds, including cannabinoid phenols, non-cannabinoid phenols (stilbenoids, lignans, spiro-indans, and dihydrophenanthrenes), flavonoids, terpenoids, alcohols, aldehydes, n-alkanes, wax esters, steroids, and alkaloids [9][10][11]. Over 500 chemical compounds have been isolated from the cannabis plant and have been reported [2]. The several classes of secondary metabolites are present in different parts of the plant with a wide range of applications (nutraceuticals, cosmetics, aromatherapy, and pharmacotherapy) that are beneficial for humans. However, previous studies have focused mainly on the cannabinoids, Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) in particular; hence, the female flower top is only harvested, while other parts of the plant are discarded [11].
Cannabinoids are a class of terpenophenolic compounds obtained by the alkylation of an alkyl-resorcinol with a monoterpene unit [12][13]. They feature alkyl resorcinol and monoterpene moieties in their molecules [12][14]. This specific chemical class in Cannabis is present in the glandular trichomes, which are abundant in the female flower as phytocannabinoid acids, and in the vegetable matrix as neutral phytocannabinoids [9][2]. They are biosynthesized by the alkylation of olivetolic acid with geranyl-pyrophosphate by a prenyltransferase to produce cannabigerolic acid (CBGA). Decarboxylation, a chemical reaction, converts the acidic forms (Δ9 THCA, CBDA, CBCA, and CBGA) into their neutral forms, which are more active and efficient in terms of pharmacological activity [10][15]. To date, 125 cannabinoids have been identified and reported, in addition to five new cannabinoids reported in the past two years, 42 non-cannabinoid phenolics, 34 flavonoids, 120 terpenoids, 3 sterols, and 2 alkaloids [10][11][2]. Terpenoids are the second largest class of cannabis compounds and are responsible for their characteristic aroma [2].
Figure 2, Figure 3 and Figure 4 below show the structures of the different classes of bioactive compounds isolated from Cannabis sativa [2][16][17].
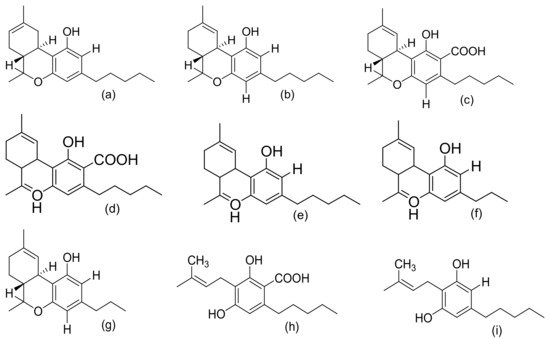
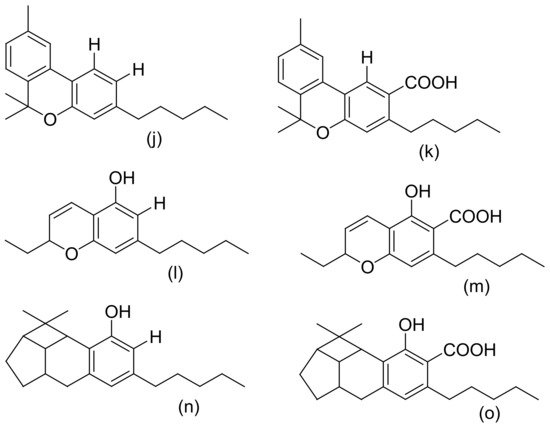
Figure 2. Chemical structures of major cannabinoids; Δ8 THC, tetrahydrocannabinol (a); Δ9-THC, tetrahydrocannabinol (b); THCA, tetrahydrocannabinolic acid (c); CBDA, cannabidiolic acid (d); CBD, cannabidiol (e); CBDV, cannabidivarin (f); THCV, tetrahydrocannabivarin (g); CBGA, cannabigerolic acid (h); CBG, cannabigerol (i); CBN, cannabinol (j); CBNA, cannabinolic acid (k); CBC, cannabichromene (l); CBCA, cannabichromenic acid (m); CBL, cannabicyclol (n); CBLA, cannabicyclolic acid (o). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
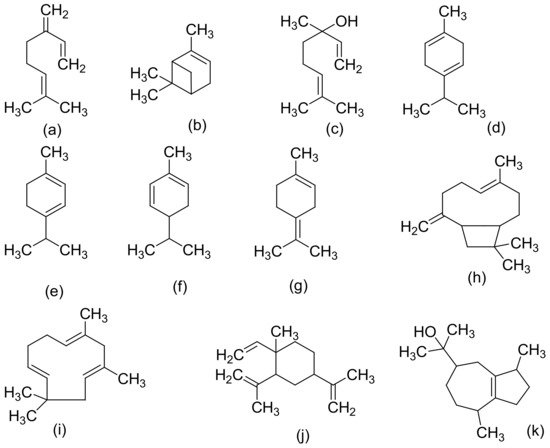
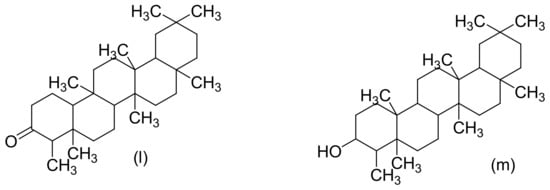
Figure 3. Chemical structures of some Cannabis sativa terpenes (Monoterpenes, Sesquiterpenes, and Triterpenoids); Mycene (a), α-Pinene (b), D-Linalool (c), Limonene (d), α-Terpinene (e), α-Phellandrene (f), α-Terpinolene (g), β-Caryophyllene (h), α-Caryophyllene (i), β-Elemene (j), Guaiol (k), Friedelin (l), and Epifriedelanol (m). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
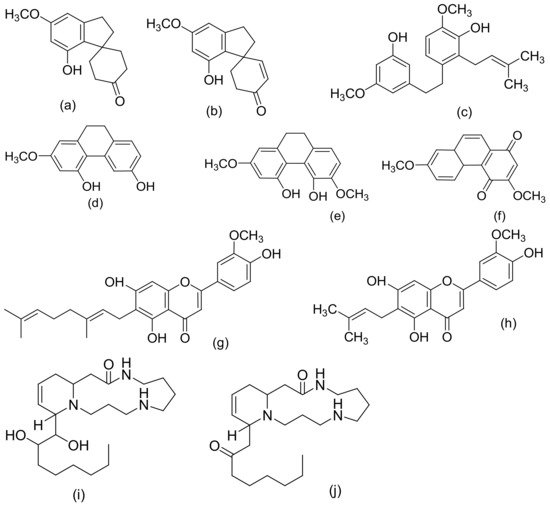
Figure 4. Chemical structures of some non-cannabinoid phenols (Spirans, phenanthrenes, flavonoids, alkaloids); Cannabispiran (a), Cannabispirone (b), Canniprene (c), Cannithrene I (d), Cannithrene II (e), Debinobin (f), Canniflavin A (g), Canniflavin B (h), Cannabisativine (i), and Anhydrocannabisativine (j). All structures drawn by Odieka, using ChemDraw Ultra 8.0.
2.2. Extraction, Isolation, and Chemical Characterization of C. sativa
Many methods have been reported for the extraction of Cannabis. These include direct maceration (DM), soxhlex extraction, ultrasound-assisted extraction (UAE), supercritical fluid extraction, and microwave-assisted extraction (MAE) [17]. However, two methods of extracting Cannabis are differentiated in the literature [17]. The first is the maceration of the plant material in an organic solvent (direct maceration) and the subsequent removal of the solvent by the concentration of the extract under reduced pressure [17]. The second is the innovative supercritical fluid extraction (SFE) method, which involves the use of pressurized solvents [17]. It is necessary for cannabinoid compounds to be extracted with organic solvents instead of water, because the active compounds are less soluble in polar solvents [17]. The most commonly used solvents are ethanol, ether, chloroform, and methanol [18]. When used for extraction, various compounds, including some undesired substances, dissolve together with the cannabinoids [18]. The high solvent power of ethanol for cannabinoid compounds is the reason why it is frequently used in home-made extracts of Cannabis [17]. However, non-desired compounds (chlorophyll, lipids, and waxy materials) are also extracted which, therefore, requires further steps to remove the co-extracted impurities for a high-purity medicinal product to be obtained [17]. A patent on the method for the isolation of herbal and cannabinoid medicinal extracts stated that the solubility of non-therapeutic substances (chlorophyll and waxy materials) is reduced when the solvent is selected from a group that includes acetonitrile, benzene, dichloromethane, diethyl ether, acetone, butanol, ethanol, chloroform, ethyl acetate, hexane, pentane, propanol, tetrahydrofuran, toluene, xylene, and various combinations of these solvents [17]. The International Conference on Harmonization (ICH) recommends the use of less toxic solvents in the manufacture of drug substances and dosage forms, and sets pharmaceutical limits for residual solvents in drug products [19]. Residual solvents pose risks to human health and are classified into three classes. Class 1 solvents (including carbon tetrachloride, benzene, and methyl chloroform) are regarded as human carcinogens and are environmentally hazardous [17]. Class 2 solvents include methanol and hexane, which are generally said to be limited, and they are possible causative agents of irreversible toxicity, such as neurotoxicity or teratogenicity [17]. Class 3 solvents (ethanol and ethyl acetate) are generally regarded as having a low toxic potential to humans [17]). Above all, ethanol is generally recognized as a safe (GRAS) solvent [17]. In a study by Brighenti et al., they compared the following four extraction techniques to obtain a high yield of medicinal cannabinoids: ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), and direct maceration (DM). They concluded that DM, with ethanol as the extraction solvent at room temperature for an overall time of 45 min, is the best extraction technique (in terms of a high yield) for non-psychoactive cannabinoids from hemp [20].
Over the last decade, compounds in Cannabis have been identified, isolated, and determined by various chromatographic techniques with different spectroscopic detection methods. C. sativa samples are analyzed for both legal and medicinal purposes [17]. Nevertheless, the knowledge of their exact composition remains very significant. In 2009, recommended methods for the identification and analysis of cannabis products were released by the United Nations Office on Drugs and Crime [21]. One notable technique that has been employed in identifying the diverse composition of the compounds found is high-performance liquid chromatography (HPLC) [17]. Spectroscopic approaches or methods are based on the variable absorbance or redirection of electromagnetic (EM) radiation by chemical bonds, resulting in the radiation or transition of the sample’s atoms to a higher energy state [22]. Some advantages are attributed to these spectroscopic methods, such as permitting spatial measurements of metabolites and offering a global metabolic fingerprint of a sample with rapid spectral acquisition [22]. Some of these approaches/methods include Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), HPLC, gas chromatography–mass spectrometry (GC–MS), and liquid chromatography–mass spectrometry (LS–MS) [17][22]. Taking into account the recommended methods and the mandatory requirement of the Ministerial decree to use only chromatographic techniques coupled with mass spectrometric detection, cannabinoid concentrations and its stability in cannabis tea and cannabis oil, prepared from standardized flowering tops obtained from the Military Pharmaceutical Chemical Works of Florence, were studied by Pacifici et al. using easy and fast ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) [23][24].
2.3. Biological Evaluation/Potentials of C. sativa
From the biological point of view, the psychoactive cannabinoids reported include Δ9 THC, cannabinol (CBN), and cannabinodiol (CBND), while cannabidiol (CBD) and other cannabinoids are non-psychoactive [10][11]. THC is the major psychoactive component and the toxicity of this metabolite of Cannabis is the most studied [11][25]. Its psychoactive component decreases in the order of inflorescence (the flower), leaves, stem, roots, and seeds, respectively [10]. The interest in the potential medical use of cannabis and cannabinoids rose significantly in the 1990s, following the discovery of the endocannabinoid (eCB) system in mammals [26]. The physiological effects of cannabinoids are exerted through various receptors, such as the cannabinoid receptors (CB1 and CB2), adrenergic receptors, and the recently discovered GPCRs (GPR55, GPR3 and GPR5) [10]. Historically, each part of the Cannabis plant is indicated mostly for pain killing, inflammation, and for mental illnesses. For example, the Cannabis root has been recommended for treating fever, inflammation, gout, arthritis, and joint pain, as well as skin burns, hard tumors, postpartum hemorrhage, difficult child labor, sexually transmitted diseases, gastrointestinal activity, and infections [27]. Cannabis has also been used to treat asthma, epilepsy, fatigue, glaucoma, insomnia, nausea, pain, and rheumatism, as well as being used as appetite stimulant and a digestive aid [26][11][2]. Since concentrations above 0.05% are pharmacologically interesting, Cannabis inflorescence and leaf material may contain sufficient cannabinoids, mono- and sesquiterpenoids, and flavonoids for therapeutic applications [11]. Cannabis terpenoids and flavonoids, mainly myrcene, limonene, pinene, β-caryophyllene, and cannflavin A, act in synergy with cannabinoids to induce pharmacological effects [26]. It was proven that these compounds, which are synthetized in the aerial parts of the plant, enhance CBD’s anti-inflammatory effects and antagonize THC dysphoric action [28]. Cannabidiol (CBD) and Cannabidavarin (CBDV) (neutral cannabinoids) have been reported to have the therapeutic potential for the treatment of epilepsy (focal seizures), as well as treating nausea and vomiting [29][30]. Conversely, THC and CBN have been found to be active in lowering intraocular pressure, and can be applied in all cases of glaucoma that are resistant to other therapies [23]. Cannflavin A and B are also notable flavonoids (prenylflavonoids) with medicinal potentials, such as their anti-inflammatory, anti-neoplastic, antioxidant, neuro-protective, anti-parasitic, and anti-viral effects [31]. Cannabis female flowering tops can be simply administered through commercially available vaporizers (e.g., Micro Vape, G Pen Herbal Vaporizer, and Volcano), buccal sprays (e.g., Sativex), oral capsules (e.g., Cannador), decoctions, or oils [26]. Only cannabis use through oral or inhalatory administration is allowed. Smoking reduces the bioavailability of cannabis ingredients by 40%, and its complete combustion can cause lung diseases and airway obstructions [26]. Homemade decoctions and pharmacy oils are currently the most widespread cannabis formulations in Europe, making the standardization of preparation difficult [26]. Cannabis pharmacological action is dose-dependent and can induce many adverse effects (AEs), principally related to THC, due to unintentional overdosing [26]. The typical symptoms of cannabis acute intoxication that have been reported are dizziness, confusion, tachycardia, postural hypotension, dysphoria, panic depression, hallucinations, allergic reactions, vomiting, and diarrhea [26][32][33]. Furthermore, withdrawal symptoms, such as irritability, aggression, anxiety, insomnia, decreased appetite, tremors, sweating, and headaches may appear after the abrupt cessation of the long-term administration of high doses of cannabis [26]. According to the ICH efficacy and safety guidelines, it is recommended to start with low doses and increase quantities after a satisfactory period of clinic evaluation, depending on the pharmacological effects and the possible adverse effects [34]. In the current COVID-19 pandemic, scientists are repurposing medicines (identifying new therapeutic use(s) of existing drugs) known for their biological potential (anti-viral or anti-inflammatory properties) to tackle the global issue and similar future viruses [35]. They have hypothesized that CBD could be used as an anti-viral agent [36] or anti-inflammatory [37][38] tool, or to inhibit pulmonary fibrosis in COVID-19 patients [39]. In addition, the known growing evidence of the anxiolytic effects of CBD have also been hypothesized to be used as a therapeutic option to treat long-lasting COVID-19-related anxiety and PTSD [40], which is likely to be a significant issue of the pandemic.References
- Farag, S.; Kayser, O. The Cannabis plant: Botanical aspects. In Handbook of Cannabis and Related Pathologies; Academic Press: Cambridge, MA, USA, 2017; pp. 3–12.
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774.
- Stevens, C.J.; Murphy, C.; Roberts, R.; Lucas, L.; Silva, F.; Fuller, D.Q. Between China and South Asia: A Middle Asian corridor of crop dispersal and agricultural innovation in the bronze age. Holocene 2016, 26, 1541–1555.
- Chandra, S.; Lata, H.; Khan, I.A.; Elsohly, M.A. Cannabis sativa L.: Botany and horticulture. In Cannabis sativa L.—Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 79–100.
- Duvall, C.S. A brief agricultural history of Cannabis in Africa, from prehistory to canna-colony. Echogéo 2019, 48, 1–25.
- Nsuala, B.N.; Enslin, G.; Viljoen, A. “Wild Cannabis”: A review of the traditional use and phytochemistry of Leonotis leonurus. J. Ethnopharmacol. 2015, 174, 520–539.
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315.
- Dutch Passion. Understanding Cannabis Phenotypes, Genotypes and Chemotypes. 2020. Available online: https://dutch-passion.com/en/blog/understanding-cannabis-phenotypes-genotypes-and-chemotypes-n980 (accessed on 19 February 2022).
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Pharm. Fluids 2020, 165, 104960.
- Lewis, M.M.; Yang, Y.; Wasilewski, E.; Clarke, H.A.; Kotra, L.P. Chemical profiling of medical Cannabis extracts. ACS Omega 2017, 2, 6091–6103.
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep. 2020, 10, 1–14.
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392.
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099.
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97.
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of medical Cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194.
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017, 80, 67–134.
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction methods, analysis, and physicochemical characterization. Stud. Nat. Prod. Chem. 2019, 66, 143–173.
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25.
- International Conference on Harmonisation (ICH). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline. Impurities: Guideline for Residual solventsq3c(R6). 2016. Available online: https://database.ich.org/sites/default/files/Q3C-R6_Guideline_ErrorCorrection_2019_0410_0.pdf (accessed on 20 January 2022).
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and hplc method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L.(hemp). J. Pharma. Biomed. Anal. 2017, 143, 228–236.
- Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products. 2009. Available online: http://www.unodc.org/documents/scientific/ST-NAR-40-Ebook.pdf (accessed on 1 November 2021).
- Allwood, J.W.; Ellis, D.I.; Goodacre, R. Metabolomic technologies and their application to the study of plants and plant-host interactions. Physiol. Plant. 2008, 132, 117–135.
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563.
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 56, 94–96.
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185.
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; del Rio, A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina 2020, 56, 237.
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216.
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for ellcytotoxic activity. Molecules 2019, 24, 3031.
- Whalley, B.; Stephens, G.; Williams, C.; Guy, G.; Wright, S.; Kikuchi, T.; GW Pharma Ltd.; Otsuka Pharmaceutical Co Ltd. Use of One or a Combination of Phyto-Cannabinoids in the Treatment of Epilepsy. U.S Patent 9,066,920, 30 June 2015.
- Parker, L.; Rock, E.; Sticht, M.; Wills, K.; Limebeer, C.L. Cannabinoids suppress acute and anticipatory nausea in preclinical rat models of conditioned gaping. Pharm. Ther. 2015, 97, 559–561.
- Erridge, S.; Mangal, N.; Salazar, O.; Pacchetti, B.; Sodergren, M.H. Cannflavins—From plant to patient: A scoping review. Fitoterapia 2020, 146, 104712.
- Rudroff, T.; Sosnoff, J.J. Cannabidiol to improve mobility in people with multiple sclerosis. Front. Neurol. 2018, 9, 183.
- Wong, K.U.; Baum, C.R. Acute Cannabis Toxicity. Pediatr. Emerg. Care 2019, 35, 799–806.
- International Conference on Harmonisation (ICH). All Guidelines. Available online: https://www.ich.org/page/ich-guidelines (accessed on 19 April 2020).
- Schlag, A.K.; O’sullivan, S.E.; Zafar, R.R.; Nutt, D.J. Current controversies in medical cannabis: Recent developments in human clinical applications and potential therapeutics. Neuropharmacology 2021, 191, 108586.
- Hill, K.P. Cannabinoids and the coronavirus. Cannabis Cannabinoid Res. 2020, 5, 118–120.
- Costiniuk, C.T.; Jenabian, M.A. Acute inflammation and pathogenesis of SARS-CoV-2 infection: Cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 2020, 53, 63.
- Byrareddy, S.N.; Mohan, M. SARS-CoV2 induced respiratory distress: Can cannabinoids be added to anti-viral therapies to reduce lung inflammation? Brain Behav. Immun. 2020, 87, 120.
- Esposito, G.; Pesce, M.; Seguella, L.; Sanseverino, W.; Lu, J.; Corpetti, C.; Sarnelli, G. The potential of cannabidiol in the COVID-19 pandemic. Br. J. Pharmacol. 2020, 177, 4967–4970.
- O’sullivan, S.E.; Stevenson, C.W.; Laviolette, S.R. Could cannabidiol be a treatment for coronavirus disease-19-related anxiety disorders? Cannabis Cannabinoid Res. 2021, 6, 7–18.
More
