Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 4 by Rita Xu.
Camel milk has recently gained the interest of consumers and the dairy industry, as it is widely suggested as an ideal substitute for cow milk. The nutritional value and the bioactivity of camel milk proteins have received particular attention from research groups and industrial companies around the world. Camel milk proteins can be used as ingredients in the manufacturing and stabilization of foods and beverages; however, in these applications, the controlled aggregation of milk proteins and stability at high temperatures and in alcohol are desirable.
- camel milk
- milk proteins
- ethanol stability
1. Introduction
Mother’s milk of different species is a high-quality source of nutrients at the early stages of life. Cow milk is the most commonly consumed and processed milk. Consumption of milk from other animals, such as camel milk, is becoming more widespread. Camel milk has recently gained the interest of consumers and the dairy industry, as it is widely suggested as an ideal substitute for cow milk [1]. Recently, the presence of alcohol (ethanol) and/or heat during the dispersion of sodium caseinate was found to have a significant effect on protein solubility but have no effect on the physical parameters of protein’s aggregations, such as size and surface charge [2].
In camel milk, despite the low ratio of casein to whey proteins, the casein fraction is mainly a substrate for bioactive peptide generation [3][4][5]. Camel milk caseins are primarily composed of β-casein, followed by α-casein and κ-casein (65%, 21%, and 3.47% of total casein, respectively) [6]. The camel milk whey proteins are predominantly α-lactalbumin, lactoferrin, camel serum albumin, peptidoglycan recognition protein, and immunoglobulins [7]. In contrast to bovine milk, β-lactoglobulin, which is a common allergen, is deficient in camel’s milk [8][9]. This deficiency, combined with the abundance of β-casein, ensures that camel milk is easier to digest and less allergenic than cow’s milk, making it tolerable for people suffering from allergic symptoms [10].
The nutritional value and the bioactivity of camel milk proteins have received particular attention from the scientific community and industrial companies around the world. Camel milk or proteins can be used as ingredients in the manufacturing and stabilization of foods and beverages; however, in these applications, controlled aggregation of milk proteins and stability at high temperatures and in alcohol is desirable. In the past, the ethanol stability of milk was used as an indicator of its freshness and to provide information on the stability of raw milk at ultra-high temperatures and powder processing [11][12]. Thus, alcohol testing was extremely useful to the global dairy industry since it allowed acidic milk, such as colostrum or mastic milk, to be processed without causing quality issues or coagulation in the dairy pasteurizer’s heating plates [13]. Much attention has been devoted to the heat stability of camel milk in recent years [14], but there is relatively little published information on the effects of ethanol on camel milk protein stability.
Ethanol stability is defined as the minimum concentration of added aqueous ethanol that results in milk coagulation. It is related to the chemical properties of milk, including pH, divalent cation content, and saline balance. The ethanol stability of cow milk is pH dependent with a typical sigmoidal pH profile; increasing the pH value of milk increases its ethanol stability.
Regarding casein fraction, the addition of ethanol to milk allows for interaction between charges on the κ-casein layer by reducing the dielectric constant of the medium [15], which decreases the negative micellar charges and their repulsion force and then promotes milk coagulation. Hence, it is relevant to know how milk proteins are destabilized by ethanol from a technological point of view. Furthermore, Horne [12] and Rosa et al. [13] reported that ethanol stability differs from one species to another. In comparison to cow milk, there is a scarcity of current understanding in the alcohol stability of camel milk. Sagar et al. [16] reported that camel milk has negative alcohol stability, though no visible flakes nor coagulation formation were reported. On the other hand, in other studies, the addition of salts and increasing pH values were found to improve the alcohol stability of camel milk to up to 85% [16][17]. Furthermore, the addition of EDTA to camel milk can improve ethanol stability [18]; moreover, it could convert sterilized camel milk from type A to B [19].
2. Chemical Composition
The chemical compositions of camel milk samples are presented in Table 1. The concentrations of fat, protein, lactose, total solids, and minerals varied significantly (p < 0.05) among camel milk samples. Fat concentration in milk ranged from 1.83% to 3.07% in camel milk samples.
Table 1. Chemical composition of camel milk from five samples—Alwatania, Alquasim, Majaheim, Wadah, and Hamra (%: g per 100 g of milk sample). a–d Different letters in the same line indicate significant differences (p < 0.05) between camel milk samples.
| Alwatania | (Darir Alabaker) Alquasim | Majaheim | Wadah | Hamra | |
|---|---|---|---|---|---|
| Fat (%) | 3.07 a ± 0.06 | 3.07 a ± 0.06 | 1.83 b ± 0.12 | 2.58 c ± 0.06 | 2.40 d ± 0.00 |
| Protein (%) | 3.23 a ± 0.06 | 3.00 b ± 0.10 | 2.87 b ± 0.06 | 2.47 c ± 0.06 | 2.43 c ± 0.06 |
| Lactose (%) | 4.87 a ± 0.06 | 4.53 b ± 0.06 | 4.37 c ± 0.06 | 4.40 bc ± 0.10 | 4.57 b ± 0.06 |
| Ash (%) | 0.78 a ± 0.01 | 0.78 a ± 0.01 | 0.78 a ± 0.01 | 0.79 ab ± 0.01 | 0.80 b ± 0.01 |
| Total solid (%) | 12.10 a ± 1.0 | 11.37 b ± 0.06 | 10.27 c ± 0.06 | 10.17 c ± 0.06 | 10.57 c ± 0.06 |
| Calcium/Cations (ppm) | 112.75 b ± 4.60 | 111.70 b ± 1.98 | 106.95 b ± 2.62 | 124.75 a ± 4.45 | 128.30 a ± 4.53 |
| Sodium/Cations (ppm) | 217.63 bd ± 11.77 | 206.06 d ± 0 | 232.78 b ± 0 | 261.18 a ± 5.16 | 188.64 c ± 2.19 |
Note: Data presented as mean ± standard deviation.
No significant differences were observed in the fat concentrations of Alquasim and Alwatania camel milk samples. However, the fat concentrations in Majaheim, Wadah, and Hamra camel milk samples were significantly lower than those in Alwatania and Alquasim camel milk, and Majaheim camel milk had the lowest values (1.83%). On the other hand, protein concentration in camel milk obtained from different camel samples varied, from 2.43% to 3.23% for Hamra and Alwatania, respectively (Table 1). Alwatania was significantly different from all other protein samples. While no significant difference was found between Alquasim and Majaheim as well as between Wadah and Hamra. As shown in Table 1, the lactose content of camel milk varied significantly (p < 0.05) across samples, ranging from 4.37% in Majaheim camel milk to 4.87% in Alwatania camel milk. The total mineral concentration (ash) of camel milk samples ranged from 0.78 to 0.80%. Significant differences in ash values were observed between different camel samples, where the Hamra sample has a greater value than the other samples (Table 1).
Table 1 shows that the values varied significantly between raw and heat-treated samples for the total solids in camel milk, ranging between 10.17% and 12.10%. Likewise, total solids of camel milk were significantly different in Alwatania and Alquasim (Darir alabaker) than other samples.
3. Calcium and Sodium Content
The sodium concentration (Table 1) in Wadah (261.18 ± 5.16 ppm) and Hamra (188.64 ± 2.19 ppm) camel milk samples were significantly different from the sodium concentration of other camel milk samples. On the other hand, the calcium content of Wadah and Hamra camel milk was higher than that of the other camel milk samples. Moreover, significant differences were found in calcium content between heat-treated and raw samples.
4. Ethanol Stability of Camel Milk Samples
4.1. Effect of NaCl
The ethanol stability results of the samples as a function of NaCl concentration are presented in Figure 1. The influence of ionic strength of NaCl on the ethanol stability of camel samples was studied by conducting experiments with solutions containing various amounts of NaCl (1–10%). As shown in Figure 1, the effect of NaCl on ethanol stability varied among camel samples. The addition of 1% (w/v) NaCl has increased the ethanol stability of camel milk from approximately 56% to 66% as shown in Figure 1. Analogously, as the concentration of NaCl increased up to 10% (w/v), the stability further improved. A similar profile was observed in Majaheim and Alwatania milk, which achieved the highest ethanol stability values (approximately 88%) of all camel milk samples regardless of sodium chloride concentration. The ethanol stability of Alquasim milk containing 7–10% (w/v) NaCl was higher than that of both Wadah and Hamra milk with the same added NaCl content. However, with the addition of NaCl between 1% and 5%, the ethanol stability was higher in Hamra camel milk (Figure 1).
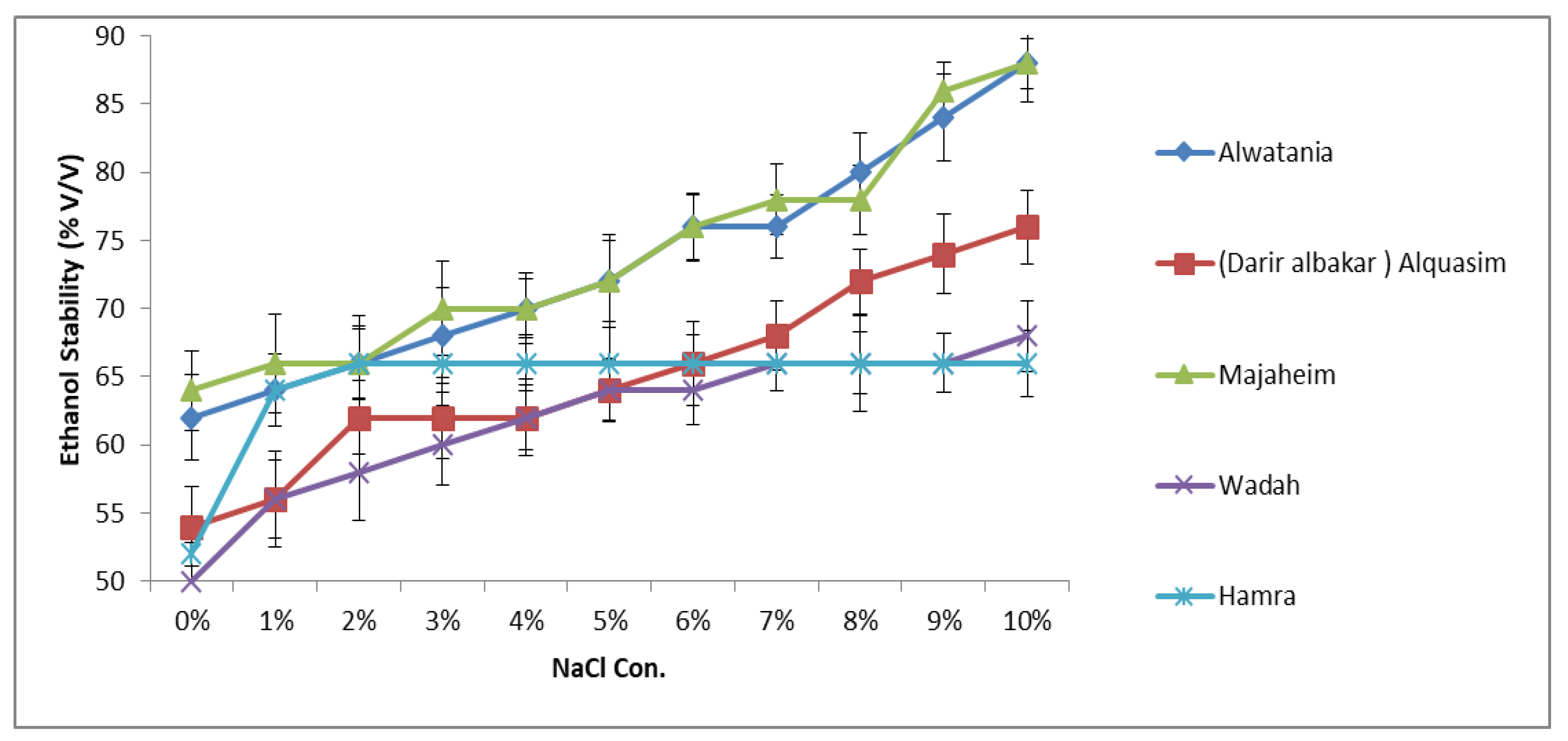

Figure 1. Effect of sodium chloride concentration (%: g/100 g) on ethanol stability of camel milk samples—Alwatania, Alquasim (Darir alabakar), Majaheim, Wadah, and Hamra (ethanol stability: % (v/v). Error bars indicate the standard deviation.
4.2. Effect of pH and Ca2+
The effects of pH and the removal of calcium (using EDTA) were studied in camel milk as shown in Figure 2. The ethanol stability of camel milk increased when the pH was increased from 5.9 to 7.1, and it was higher for EDTA-treated milk than control milk (Figure 2). For camel milk from the Majaheim and Hamra breeds (Figure 2C,E), the stability was similar between the control and EDTA-treated milk, regardless of the pH value, except at a pH of 6.4, 6.7, and 6.9 for Hamra milk, where EDTA-treated milk presented higher ethanol stability than the control (74%, 96%, and 100%, respectively). Contrastingly, EDTA-treated milk in the Wadah milk sample (Figure 2D) exhibited greater ethanol stability values than control milk. For Alquasim milk (Darir alabakar), the ethanol stability values of EDTA-treated milk (Figure 2A) were higher when compared to control milk. However, at a pH level above 6.8, the control had the highest ethanol stability values (78%) compared to EDTA-treated milk. The stability of Alwatania camel milk (Figure 2B) increased when the pH increased and was considerably higher for EDTA-treated milk than control milk. The Alwatania camel milk sample demonstrated larger ethanol stability differences compared to milk from other heat-treated camel samples (Figure 2A), suggesting that Alwatania milk is more sensitive to EDTA treatment.
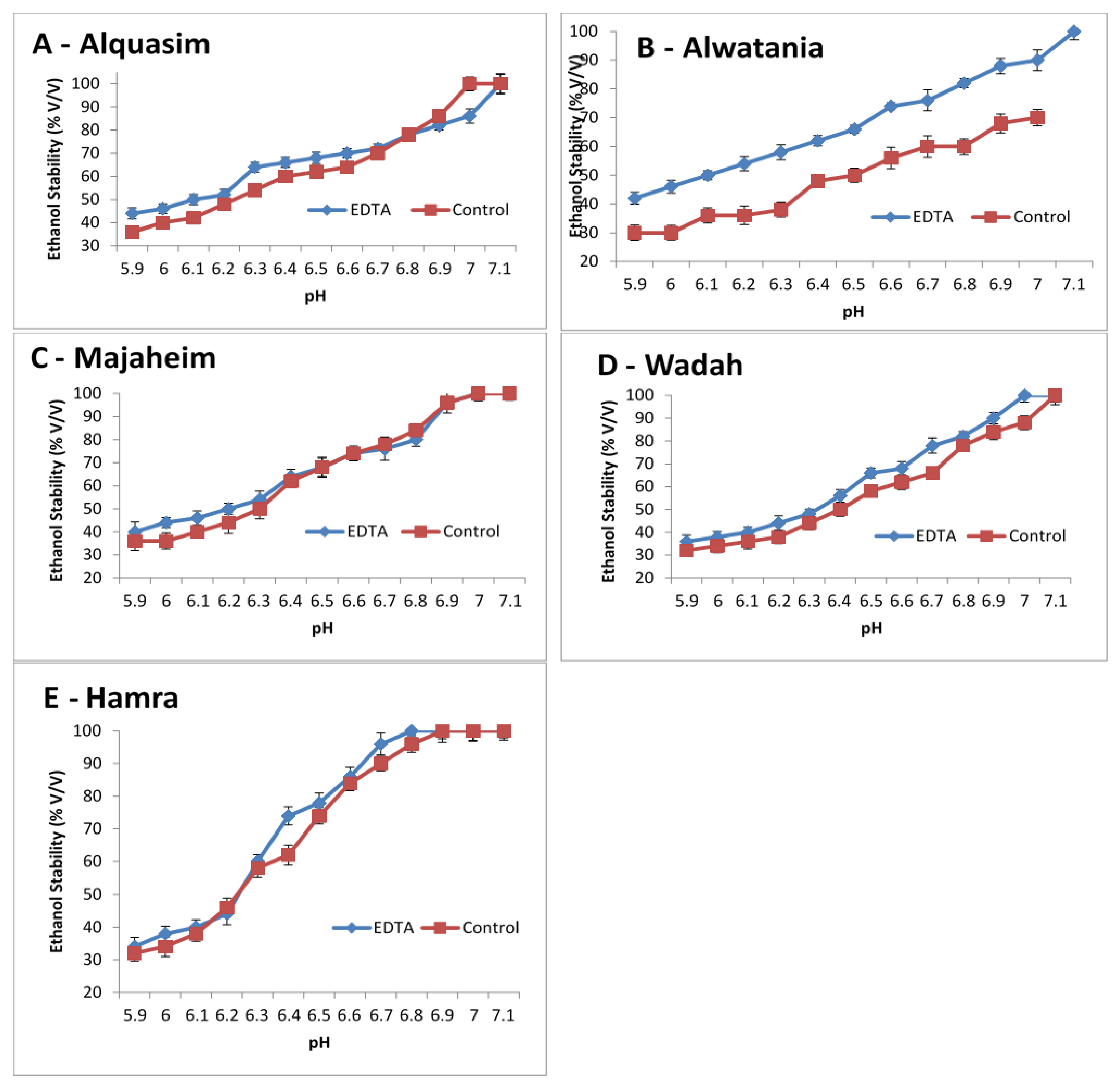

Figure 2. Changes in pH and ethanol stability of camel milk samples, Alquasim (A), Alwatania (B), Majaheim (C), Wadah (D), and Hamra (E) (ethanol stability: % (v/v)). Error bars indicate the standard deviation.
References
- El Hatmi, H.; Jrad, Z.; Oussaief, O.; Nasri, W.; Sbissi, I.; Khorchani, T.; Canabady-Rochelle, L.L.S. Fermentation of dromedary camel (Camelus dromedarius) milk by Enterococcus faecium, Streptococcus macedonicus as a potential alternative of fermented cow milk. LWT 2018, 90, 373–380.
- Lin, Y.; Kelly, A.L.; O’Mahony, J.A.; Guinee, T.P. Fortification of milk protein content with different dairy protein powders alters its compositional, rennet gelation, heat stability and ethanol stability characteristics. Int. Dairy J. 2016, 61, 220–227.
- Jrad, Z.; El Hatmi, H.; Adt, I.; Khorchani, T.; Degraeve, P.; Oulahal, N. Antimicrobial activity of camel milk casein and its hydrolysates. Acta Aliment. 2015, 44, 609–616.
- Alshuniaber, M.; Alhaj, O.; Abdallah, Q.; Jahrami, H. Effects of camel milk hydrolysate on blood pressure and biochemical parameters in fructose-induced hypertensive rats. Nutr. Food Sci. 2021, 52, 292–307.
- Lajnaf, R.; Gharsallah, H.; Attia, H.; Ayadi, M.A. Comparative study on antioxidant, antimicrobial, emulsifying and physico-chemical properties of purified bovine and camel β-casein. LWT 2021, 140, 110842.
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25.
- Lajnaf, R.; Picart-Palmade, L.; Cases, E.; Attia, H.; Marchesseau, S.; Ayadi, M.A. The foaming properties of camel and bovine whey: The impact of pH and heat treatment. Food Chem. 2018, 240, 295–303.
- El-Hatmi, H.; Girardet, J.M.; Gaillard, J.L.; Yahyaoui, M.H.; Attia, H. Characterisation of whey proteins of camel (Camelus dromedarius) milk and colostrum. Small Rumin. Res. 2007, 70, 267–271.
- Lajnaf, R.; Zouari, A.; Trigui, I.; Attia, H.; Ayadi, M.A. Effect of different heating temperatures on foaming properties of camel milk proteins: A comparison with bovine milk proteins. Int. Dairy J. 2020, 104, 104643.
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and therapeutic perspectives of camel milk and its protein hydrolysates: A review on versatile biofunctional properties. J. Funct. Foods 2019, 60, 103441.
- Chen, B.; Lewis, M.J.; Grandison, A.S. Effect of seasonal variation on the composition and properties of raw milk destined for processing in the UK. Food Chem. 2014, 158, 216–223.
- Horne, D.S. Ethanol stability and milk composition. In Advanced Dairy Chemistry; McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 225–246.
- Pinto da Rosa, P.; Pio Ávila, B.; Damé Veber Angelo, I.; Moreira da Silva, P.; Garavaglia Chesini, R.; Nessy Mota, G.; Aristimunho Sedrez, P.; Albandes Fernandes, T.; Bugoni, M.; Fernando Buttow Roll, V. Factors That Affect the Thermal Stability of Bovine Milk and the Use of Alcohol Test in the Milk Industry—A Review. Nucl. Anim. 2020, 12, 15–46.
- Zhang, B.Y.; Xu, S.; Villalobos-Santeli, J.A.; Huang, J.-Y. Fouling characterization of camel milk with comparison to bovine milk. J. Food Eng. 2020, 285, 110085.
- Ye, R.; Harte, F. Casein maps: Effect of ethanol, pH, temperature, and CaCl2 on the particle size of reconstituted casein micelles. J. Dairy Sci. 2013, 96, 799–805.
- Sagar, S.P.; Mehta, B.M.; Wadhwani, K.N.; Darji, V.B.; Aparnathi, K.D. Evaluation of camel milk for selected processing related parameters and comparisons with cow and buffalo milk. Int. J. Health Anim. Sci. Food Saf. 2016, 3, 27–37.
- Metwalli, A.A.; Hailu, Y. Effects of Industrial Processing Methods on Camel Milk Composition, Nutritional Value, and Health Properties. In Handbook of Research on Health and Environmental Benefits of Camel Products; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 197–239.
- Zhao, D.; Bai, Y.; Mutu, J.; Zhang, H. Ethanol stability of Alxa bactrian camel milk. J. Camel Pract. Res. 2010, 17, 189–193.
- Alhaj, O.A.; Metwalli, A.A.M.; Ismail, E.A. Heat stability of camel milk proteins after sterilisation process. J. Camel Pract. Res. 2011, 18, 277–282.
More
