Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Miguel Ángel Olego and Version 2 by Catherine Yang.
Vineyard calcareous soils are usually low in organic matter, which makes them prone to physical, chemical, and biological degradation. Besides, these soils are also usually poor in various nutrients in plant-available form, e.g., iron. To make up for this lack of soil fertility, on the one hand, manures, and on the other, iron chelates are usually used. However, the soil application of these materials is not free from problems, and other amendments based on leonardites could be advantageously used as an alternative.
- ferric chlorosis
- grapevine
- leonardite
- soil organic matter
- calcareous soils
- organic amendments
- vineyard soils
- vine nutrition
1. Introduction
There are three core soil properties (texture, mineralogy, and soil organic matter (SOM)) that constitute the natural capital of soils [1]. Soil texture and mineralogy are inherent properties of soil, inherited from bedrock, and slowly evolve along years, whilst SOM levels dramatically change with land use and management [1]. Despite the small percentage that SOM represents in most soils [2], it improves the buffering and cation exchange capacity in soils, having a high heavy metal adsorption and complexing capacity. A decrease of SOM can lead to the drastic impairment of soil physical and chemical properties, with negative impacts on soil nutrient cycling mechanisms [3] as well as on crop production. Therefore, the quality of soils determines agricultural sustainability and environmental quality [4], but the impact of SOM and its humic substances originated by humification on crop productivity is quite complex because it affects a range of soil properties, not just a single one [5]. These reasons reflect the importance of SOM as a soil component, which can be summarised according to its important influence on soil quality.
The SOM benefits to grapevines are indirect: influences water retention and permeability as well as aggregate structure, nutrient availability, and buffering capacity [6]. Moreover, in order to maintain profitable grape production sustainability in many situations, the use of external inputs, such as chemical fertilizers, should be lowered and SOM contents increased [7]. For most vineyard soils, the ideal SOM content is in the range 1–3% [8]. In this sense, White and Krstic [9] provide optimal values for soil organic carbon based on soil textural classes. However, the right amount of SOM in a vineyard is a function of land management decisions like the amount and weight of traffic or the presence or absence of cover crops [10]. The soil management of Mediterranean vineyards, despite their low organic matter content, usually consists in continuous tillage maintaining bare soils [11]. Such intensive working of vineyard soils can lead to soil degradation, with loss of soil fertility, acceleration of soil erosion, SOM mineralization, and hence increased CO2 emission [3] and nutrient loss [11]. Specific vineyard soil properties like coarse textural classes can lead to higher SOM losses. Thus, the maintenance of appropriate SOM levels becomes essential to sustain the Mediterranean viticulture, as it is also critical to maintain high agriculture productivity [12]. However, although a continuous supply of organic material must be met to maintain an appropriate level of SOM in vineyard soils, we must focus on the principles of efficient and ecologically sound management of SOM with organic fertilisers.
Worldwide, manures have been spread on arable soils over the years [13][14][13,14] as an important supply of organic matter, useful microorganisms, and plant nutrients [15]. However, the role of manure in soil fertility and SOM maintenance involves some potential environmental drawbacks, such as water pollution as a consequence of high surface nutrient concentrations (N and P) [16], or increases of toxic heavy metals in soil surface layers [15][17][18][15,17,18]. This could be of high prominence for Cu as it is widely used as an additive in most animal feeds [18]. An inadequate manure management can lead to dispersion of clay colloids, fostered by K+, Na+, and NH+4 accumulation, which is associated with large applications of manure [19], and at the same time a decrease of soil stability in vineyards [20]. Additionally, in the last 30 years, the occurrence of antibiotics and hormones, both in raw or treated livestock manures (swine, cattle, poultry and horse), has been widely reported [14]. Moreover, Gravert et al. [21] found steroids in agricultural soils amended with cattle manure, whereas Kjær et al. [22] warned about the contamination risk to the aquatic environment due to the leaching of steroid hormones, potential endocrine disruptors, from swine manure-treated fields. Finally, livestock manure may also contain large numbers of pathogenic microorganisms, which can potentially pose risks to human health [15].
Therefore, to maintain a sustainable agriculture practice meeting environmental regulations, alternative organic fertilizer sources should be found. One alternative that may be used as a substitute of manures is leonardite. Originating from an oxidation product of lignite related to subsurface mining or as sediments enriched with humic compounds [23], leonardite is a concentrated form of humic and fulvic acids [24], and its application has been shown to improve crop nutrient uptake [23][25][23,25]. Because humic acids along with fulvic acids are essential components of soil organic matter, playing a critical role in improving soil properties [26], it is expected that the use of leonardite directly and leonardite-derived humic substances as soil amendments and plant stimulants will improve the physicochemical and biological aspects of soil, promoting plant yield and quality [25][27][28][25,27,28]. Despite the above, previous research has focused on identifying and evaluating the effects of leonardite on soil trace element mobility [29][30][31][32][29,30,31,32], soil microbiome structure and functionality [24][28][30][24,28,30], stability of soil aggregates [33], as well as crop tolerance to soil drought [23] and salinity [34]. However, less research has been performed on how this organic material affects grapevine nutrition and harvest 5uality.
2. Effects of Leonardite Amendments on Vineyard Calcareous Soil Fertility, Vine Nutrition and Grape Quality
According to the amendments’ rates and compositions, as well as the method of application, SOM increased each year in 0.064% and 0.067% following the addition at the onset of crop season of, respectively, the leonardite-alone amendment and the leonardite-plus-FSH one. However, at the end of the season, SOM was found to be in each treatment, respectively, 0.10% and 0.45% higher than the control on average. Therefore, most of the observed increase in SOM cannot be due to a simple transfer of organic matter mass from the amendments to the soil environment. Besides, the leonardite amendments did not only work as a supply of organic matter to the soil, but also of several nutrients. This can make a difference for plant nutrition regarding the control in a soil as nutrient-depleted as this, to which no other fertilizers were applied. Although this difference for plant nutrition could only be confirmed in the plant on a statistical basis for P-p, interesting trends for higher petiole nutrient contents could be observed for K-p, Fe-p, Mn-p, and Zn-p, overall, with the leonardite-plus-FSH amendment. In agricultural systems, in general, 70% of SOM can be traced back to plant roots and 30% to the aerial plant organs, including the incorporated crop residues [35][36][37,38]. Since in the study site weed growing on the lanes between plants is systematically prevented, and since vine crop residues are not incorporated, the most important source of organic matter to the soil are the vine roots. Therefore, it can be hypothesized that the higher nutrient content in the amended soil area fostered root growth in that soil part, and the subsequent organic rhizodeposition effectively contributed to the observed SOM increase by providing fresh organic material to the soil organisms. Such an increase in SOM as a consequence of the addition of leonardite was also found in other works [24][27][24,27]. Besides, the higher values of extracted Fe and Zn obtained in subplots fertilized with leonardites agree with Maqueda et al. [37][39], even though those obtained for Cu and Mn are not in agreement with them. In the present work, however, one leonardite treatment was supplemented with an extra source of nutrients, i.e., the FSH, and this supplemented treatment remarkably increased, although non-significantly, the SOM content regarding the leonardite-alone treatment. It is important to note that this FSH-supplemented treatment contributed only 2–15% of soil increments of most macronutrients regarding the leonardite-alone, but remarkably higher increments of micronutrients. These higher nutrient contributions by the leonardite-plus-FSH treatment were reflected in the plant-available soil P content, which featured a remarkable, though non-significant, increase regarding the leonardite-alone. However, this was reflected in the petioles P content, which featured a significant increase from the leonardite-alone treatment to the leonardite-plus-FSH one. Regarding K and the micronutrients, the increment of their plant-available soil contents from the leonardite-alone to the leonardite-plus-FSH treatment was also observed, although only significantly for Mn. Interestingly, the same trends of higher petiole contents of K and the micronutrients under the leonardite-plus-FSH treatment regarding the leonardite-alone treatment were observed. The rise in the plant-available soil contents of all the aforementioned nutrients from the leonardite-alone to the leonardite-plus-FSH treatment can be explained on the basis of quantity, i.e., as indicated the leonardite-plus-FSH contributed higher nutrient amounts. However, the rise in the plant available soil contents under the leonardite FSH-supplemented treatment could be explained also on the basis of intensity. This would be due to the significant soil pH drop of 0.2 units under the leonardite-plus-FSH treatment. Regarding the intensity effect of soil pH, this is recognized as the most relevant soil property in controlling element availability for plant nutrition [2][38][2,40]. Specifically, in the soil pH range 8.0–8.5, the plant-availability of P, Mn, Cu, and Zn increases as soil pH decreases [39][41], and it contributes to explaining the trend towards higher petiole contents for all these elements. Moreover, Ece et al. [40][42] also found a similar significant difference in plant P when applying leonardites to a slightly basic soil, low in SOM, and loamy-textured. Besides, both [23][41][23,43] also showed a better P crop nutritional status in their field trials with the combined application of sulphur and leonardites in corn and leonardite alone in cherry, respectively. The findings of these authors are thus in agreement with the ones obtained in the present work. Therefore, the increased petiole P content in the present study suggests that both leonardite treatments could reduce soil P fixation while increasing its plant availability content, and the leonardite FSH-supplemented somewhat more than the leonardite-alone one. Therefore, since P is directly linked to the plant growth rate and energy transfer in plants, among other important metabolic processes [23], these leonardite treatments can alleviate the P deficiency in calcareous vineyard soils. In order to understand why the leonardite-plus-FSH was able to significantly decrease soil pH, one can start from the fact Fe(II) in the ferrous sulphate heptahydrate is very labile in well-aerated high-pH soils [42][44] like the one in the study site. Under these conditions of well aeration and high soil pH, Fe(II) abiotically reacts with O2 to give Fe(III) which, in turn, experiences hydrolysis to precipitate as Fe(OH)3 while releasing 3 mols of H+ to the soil environment per each mol of precipitated Fe(III). The overall reaction is as follows: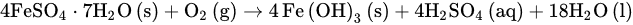 4FeSO4⋅7H2O(s)+O2(g)→4Fe(OH)3(s)+4H2SO4(aq)+18H2O(l)
4FeSO4⋅7H2O(s)+O2(g)→4Fe(OH)3(s)+4H2SO4(aq)+18H2O(l)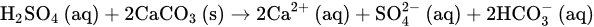 H2SO4(aq)+2CaCO3(s)→2Ca2+(aq)+SO2−4(aq)+2HCO−3(aq)
H2SO4(aq)+2CaCO3(s)→2Ca2+(aq)+SO2−4(aq)+2HCO−3(aq)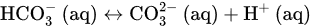 HCO−3(aq)↔CO2−3(aq)+H+(aq)
HCO−3(aq)↔CO2−3(aq)+H+(aq)