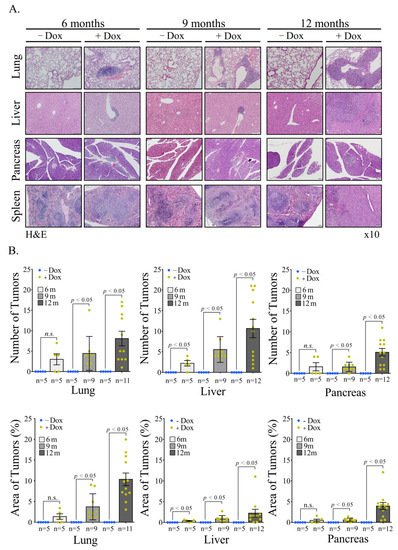
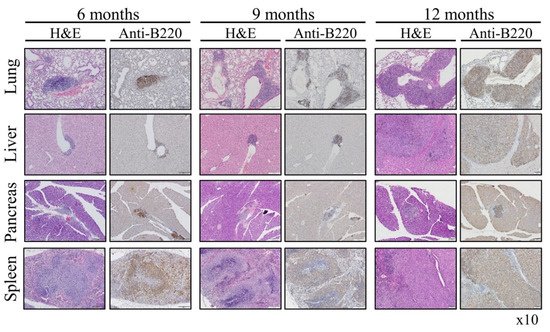
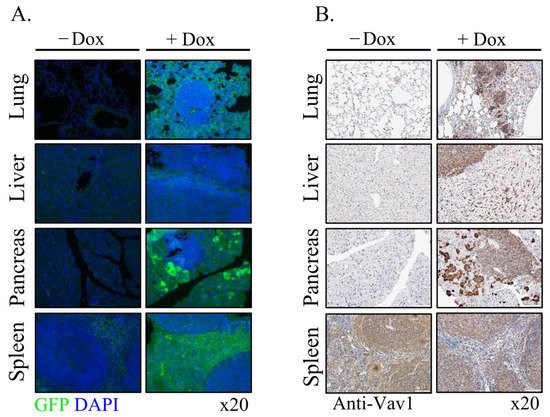
Host defense, inflammation, organogenesis, tissue repair, cancer growth, and immunity are all regulated by a complex network of epithelial cells and leukocytes. Bidirectional interactions, rather than the functions of individual cell types, contribute to tissue integrity and immunological homeostasis under steady-state conditions, while they can produce a complex pathologic tissue microenvironment leading to disease development. Indeed, the question then arises as to is how can the expression of Vav1 in one histologic compartment, the epithelium, lead to the development of tumors of a different histologic origin, B-lymphomas. One possible example is that of Mucosa-associated lymphoid tissue (MALT) lymphomas that originate in sites of chronic epithelial inflammation in various sites of the body, where it plays a role in regulating mucosal immunity
[35][41]. MALT lymphomas are present in the gastrointestinal tract
[36][42], nasopharynx
[37][43], thyroid
[38][44], breast
[39][45], lung
[40][46], salivary glands
[41][47], eye
[42][48], and skin
[43][49] and exhibit characteristics with B cells located in the marginal zone of lymph node follicles. MALT lymphomas originate in sites of chronic epithelial inflammation. One example are MALT lymphomas in the stomach which are commonly caused by
Helicobacter pylori infection
[36][42]. The association between epithelial cells and lymphoma generated is best explored in patients with primary Sjögren’s syndrome (pSS), which is characterized by chronic hyperactivation of B lymphocytes, that exhibit an increased risk of development of non-Hodgkin lymphoma
[36][42]. Salivary gland epithelial cells (SGECs) were shown to play a role in promoting B cell activation, differentiation and survival through direct interaction and cytokine production
[36][41][44][42,47,50]. These cytokines include IL-6, B-cell activating factor (BAFF), and type I interferon leading to B cell activation, homeostasis, and survival
[45][46][47][48][49][50][51,52,53,54,55,56]. SGECs were shown to express immune-competent molecules that regulate lymphocyte recruitment, homing, activation, differentiation, and survival
[51][57]. Thus, the crosstalk between SGECs and B cells suggests that salivary gland epithelial cells play a critical role in SS pathogenesis. The activation of Vav1 by various pathogens, such as Helicobacter pylori
[52][58] and mycoplasma
[53][59], that may drive MALT carcinogenesis was also suggested, yet it is not clear whether the B-cell lymphomas developed in Rosa Vav1 mice fit the same pathological classification.
One of the likeliest possibilities that B-cell lymphoma develop in Rosa Vav1-transgenic mice is that Vav1-epithelial expressing cells secrete ligands that affect B-cell proliferation. Indeed,
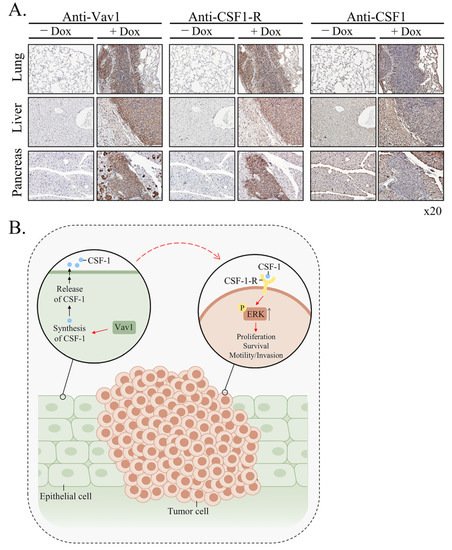
theour results clearly demonstrate the increased expression of CSF-1 in epithelial cells, while the expression of its receptor is found on the lymphoma cells (
Figure 46). Vav1 was shown to be involved in increased secretion of ligands which function in an autocrine or paracrine fashion. Thus, the human mammary epithelial cell line MCF-10A, which ectopically expresses an oncogenic form of Vav1, exhibits increased migration and morphological changes, accompanied by secretion of an autocrine EGF receptor ligand
[54][60]. Also, diminished Vav1′s expression in lung cancer cell lines reduced the expression of the growth factors, TGFα and EGF
[54][55][60,61], and CSF-1
[56][30], which led to reduced tumorigenicity. Depletion of CSF1 in lung cancer cells led to decreased proliferation and focus-formation in vitro, as well as diminished tumor growth in immune-compromised mice, suggesting that CSF-1 secretion is cardinal for tumorigenicity
[56][30]. Immunohistochemical analysis of Vav1 and CSF-1 expression of primary human lung tumors pointed to a strong link between these proteins, associated with tumor grade.
ThWe
researchers aalso demonstrated that conditioned media from lung cancer cells contain a growth factor, potentially CSF-1, that activates U937 monocytic cells, and that conditioned media from U937 cells instigates signaling in lung cancer cells, thus suggesting a cross-talk mechanism between immune cells and lung cancer cells mediated by CSF-1. Thus, Vav1 could influence tumor growth and the tumor’s microenvironment via CSF-1 in an autocrine/paracrine mechanism. Such a potential mechanism was also shown for breast and ovarian carcinomas. Thus, the rate of tumor progression is reduced substantially in CSF-1-knockout mice
[57][62]. Macrophages expressing EGF promote migration and invasiveness of breast carcinoma cells as well as CSF-1 expression by the latter, and cancer cell-derived CSF-1 is able to induce EGF production in macrophages
[58][63]. Thus, cytokines/growth factors such as CSF-1 can mediate the interaction between epithelial tumor cells and inflammatory cells.
CSF-1 contributes to the survival, proliferation, and differentiation of mononuclear phagocytes and the female’s reproductive tract
[59][64]. Under physiological conditions, CSF-1 is produced by fibroblasts, endothelial cells, monocytes, macrophages, osteoblasts, microglia, keratinocytes, bone marrow stromal cells, natural killer cells, B-cells and T-cells, and epithelial cells
[60][61][65,66]. It is an essential regulator of development and homeostasis of the mononuclear phagocyte system and, by extension, a key factor of CSF1-dependent macrophage control of development and homeostasis
[59][62][63][64,67,68]. CSF-1 appears to play an autocrine and/or paracrine role in cancers of the ovary, endometrium, breast, lung, nervous system, and myeloid and lymphoid tissues, within which overexpression of CSF-1 receptor is considered as a prognostic factor for survival in cancer
[63][68].
TheseOur results demonstrating the presence of CSF-1 in the epithelia of the various organs of Rosa Vav1 transgenic mice that develop B-cell lymphoma, points to the possibility that it can be produced in cells other than the hematopoietic system, once signaling driven by proteins such as Vav1 are abnormally expressed (
Figure 46). In the hematopoietic system, CSF-1 exerts its pleiotropic effects by binding to a single class of high-affinity receptors (CSF-1R) expressed predominantly on monocytes, macrophages, and their committed BM precursors
[62][67]. Based on this knowledge,
thwe
researchers eexpected the CSF-1 receptor to be expressed on monocytes within the B-Cell lymphomas identified in the Rosa Vav1 transgenic mice. However, no staining was detected when
the researchers we used F4/80 antibodies that usually bind to macrophages from different sites including the peritoneal cavity, lung, spleen, and thymus, to blood monocytes and to macrophages derived from bone marrow precursors in culture (data not shown)
[64][69], thus suggesting that the marked expression of CSF-1 receptor noted by
the researcherus is expressed on other cells (
Figure 46). Although CSF-1R expression is generally thought to be restricted to myeloid lineage cells, recent studies convincingly demonstrated its aberrant expression on non-myeloid lineage cells, including malignant B cells and classic Hodgkin lymphoma
[65][66][67][68][69][70,71,72,73,74].
Figure 46. Mechanism of generation of B-cell Lymphomas in Rosa Vav1 mice. (A) Sections of lung, liver, and pancreas from Rosa Vav1 mice either treated (+Dox) or non-treated (−Dox), 12 months post transgene induction stained with anti-Vav1, anti-CSF-1R, and anti-CSF1 antibodies are depicted. Representative pictures are shown. Magnification at ×20. (B) The model proposed for the generation of B-cell lymphomas due to the aberrant expression of Rosa Vav1 in epithelial cells suggests that CSF-1 secretion from the epithelial compartment activates the CSF-1R on B-cells, leading eventually to the development of B-cell lymphoma.
The emerging model from
the our
esearchers' studies depicted in
Figure 46B is that aberrant expression of Rosa Vav1 in epithelial cells leads to CSF-1 secretion (
Figure 46A). CSF-1, in turn, activates the CSF-1R on B-cells and leads to the enhancement of ERK phosphorylation and cell propagation, leading eventually to the development of B-cell lymphoma. The fact that the mere expression of Vav1 in epithelial cells does not lead to the development of carcinomas further substantiates
the our
esearchers' previous studies with Vav1-pancreatic transgenic mice
[22]. One would have expected, based on
the reseaour
chers' current results, that Vav1 will lead to the generation of B-Cell lymphomas when expressed in the pancreas, but it’s expression in both studies was under different promoters, rosa26 in this
researchstudy versus Ptf1a promoter in the pancreas, which might affect its expression and influence. Yet, it is obvious that Vav1’s ectopic expression impacts tumor development either in the pancreas when it is co-expressed with mutant K-Ras or when it is expressed in various tissues under the Rosa promoter.