Heat is a kinetic process whereby energy flows from between two systems, hot-to-cold objects. In oro-dental implantology, conductive heat transfer/(or thermal stress) is a complex physical phenomenon to analyze and consider in treatment planning. Hence, ample research has attempted to measure heat-production to avoid over-heating during bone-cutting and drilling for titanium (Ti) implant-site preparation and insertion, thereby preventing/minimizing early (as well as delayed) implant-related complications and failure.
In Tthe present work, our goal is two-fold: (A) We propose to solve the standard heat equation is proposed to be solved, modifying the imposed boundary conditions without any additional source term, and on the other hand, (B) we fill a gap is filled in the literature via obtaining an exact analytical solution of a somewhat simplified problem, which nevertheless, encapsulates the physics and reproduces the results already found in previous works via numerical analyses. In addition, for the first time, thwe intrinsic time is introducedoduce and involved herein, the intrinsic time, a “proper” time that characterizes the geometry of the dental implant fixture and overall system, and how we show how the interplay between that time and the exposure time influences temperature changes, and subsequent implant survival, are shown. Thus, this work aims to complement the overall clinical diagnostic and treatment plan for enhanced biological one–implant interface and mechanical implant stability and success rates, whether for immediate or delayed implant loading strategies.
1. Introduction
Despite significant progress in the diagnosis, prevention, management, and treatment of oro-dental diseases, teeth and supporting tissues, either damaged or lost due to disease or trauma, continue to embody a burden. The quality of life of men and women of all ages worldwide is affected by a missing tooth in several ways. Speaking difficulties, pain, loss of confidence, and poor eating (chewing/mastication/deglutition) capacity, are only a few aspects of this. Thus, a reduction in number of teeth may deteriorate quality of life (QoL)
[1]. Moreover, several previously-reported articles stated that missing teeth are closely related to death
[2][3][4][2,3,4]. Nowadays, a number of options exist for the replacement of missing teeth. Dental implants are used to replace missing, lost, or extracted teeth
[5], a great option for patients missing (partially or fully) natural teeth/dentition, because they act as a secure anchor for artificial replacement teeth and/or prosthetics and eliminate the instability associated with surface adhesives and removable bridges
[6]. Hence, dental implants, Titanium-based mainly, are a predictable treatment option/modality for the rehabilitation of partially- and completely-edentulous patients. Further, when compared to traditional bridges and dentures (removable solutions), implant-retained prostheses (fixed solutions) might tend to be easier to maintain and could require fewer re-visits to the dental clinic.
Indeed, dental implant use has nearly tripled since its introduction in 1986
[7], and it is expected to continue to rise or grow, rapidly. People of all ages are turning to dental implants to replace a single tooth, several teeth, or a full set of dentures. The leading reasons for choosing or preferring dental implants are: to restore normal mastication/eating (and choice of foods), speaking and laughing, to enhance facial appearance, smile and confidence (increase self-esteem and reduce self-consciousness), and to increase denture retention (via improving the support to facial muscles). Dental implants changed (and continue to change) the way people live; they are re-discovering the comfort and confidence to chew, eat, speak, smile, laugh, socialize, and enjoy life overall; indeed, this has a positive impact on QoL
[6].
Due to consumption of hot foods and liquids, the human tooth is daily subjected to thermal loading. Heat generated on the tooth surface from intra-oral temperature changes is transferred via conduction through the enamel, dentin, and pulp. Since enamel and dentin have lower values of thermal conductivity, the pulp is protected against rapid thermal fluctuations
[8]. The thermal behavior, however, of restored teeth is significantly different in comparison to intact teeth, as the metals used in clinical restorative applications, such as titanium or titanium alloy, are excellent thermal conductors
[9][10][11][12][13][14][9,10,11,12,13,14]. High temperatures may cause irreversible damage to tissues and organs
[15], while the habitual consumption of extremely hot foods and beverages may affect implant treatment modality. Mechanical stability of dental implants is a prerequisite for successful rehabilitative and restorative therapy, and furthermore, it can be stated that the cornerstone of successful dental implant therapy is an intact biological osseointegration around the implant (fixture), thereby playing an important role in provision of the pursued stability. Osteoblast cells require in situ activation to increase bone density and establish high anchorage and subsequent high stability, survival, and success of the implant
[16]. Thermal injury to the implant–bone interface may lead to bone necrosis and loss of osseointegration. Previous studies have shown that osteoblasts may be severely damaged by a thermal impulse of 42 degrees (10-minute heat shock)
[17], and that some bone proteins are lost
[18]. Furthermore, it was stated that the temperature threshold for necrosis of the bone (cortical) is 47 degrees (for 1 min)
[19][20][21][19,20,21]. Yet, the literature reports intra-oral temperatures reaching 67–77 degrees during function and during the consumption of hot water/liquids
[22][23][22,23].
Intra-bony heat generation, during surgical implant insertion, is another story (alarming), with few serious reports on temperatures at the implant–bone interface, whether during and post-surgical preparation, and/or during and post-hot substance consumption. Questions pertinent to threshold level(s) and probable transient changes in osteoblasts are raised. Thus, the transient heat transfer under thermal load is of
vital significance in dentistry, in general, and in practical oro-dental implantology, in particular. In the literature, there already exist some previous works on the subject, where the authors attempted to model and investigate the effects of “thermal load(s)” on the bone–implant interface system
[24][25][26][27][28][24,25,26,27,28] (see e.g.,
[29][30][29,30] for heat transfer from warm water to foot in a footbath).
2. Thermal Load and Heat Transfer
Formulation of the Physical Problem
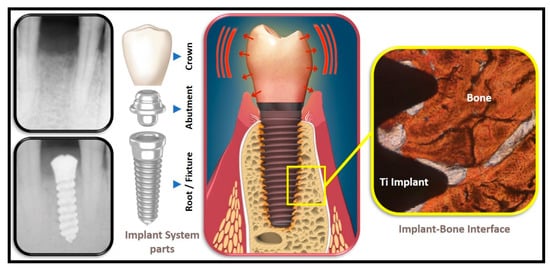
The dental implant system, typically, consists of three main parts, namely the root or fixture (good conductor), the abutment (good conductor) as well as the crown (moderate to poor conductor) (see
Figure 1).
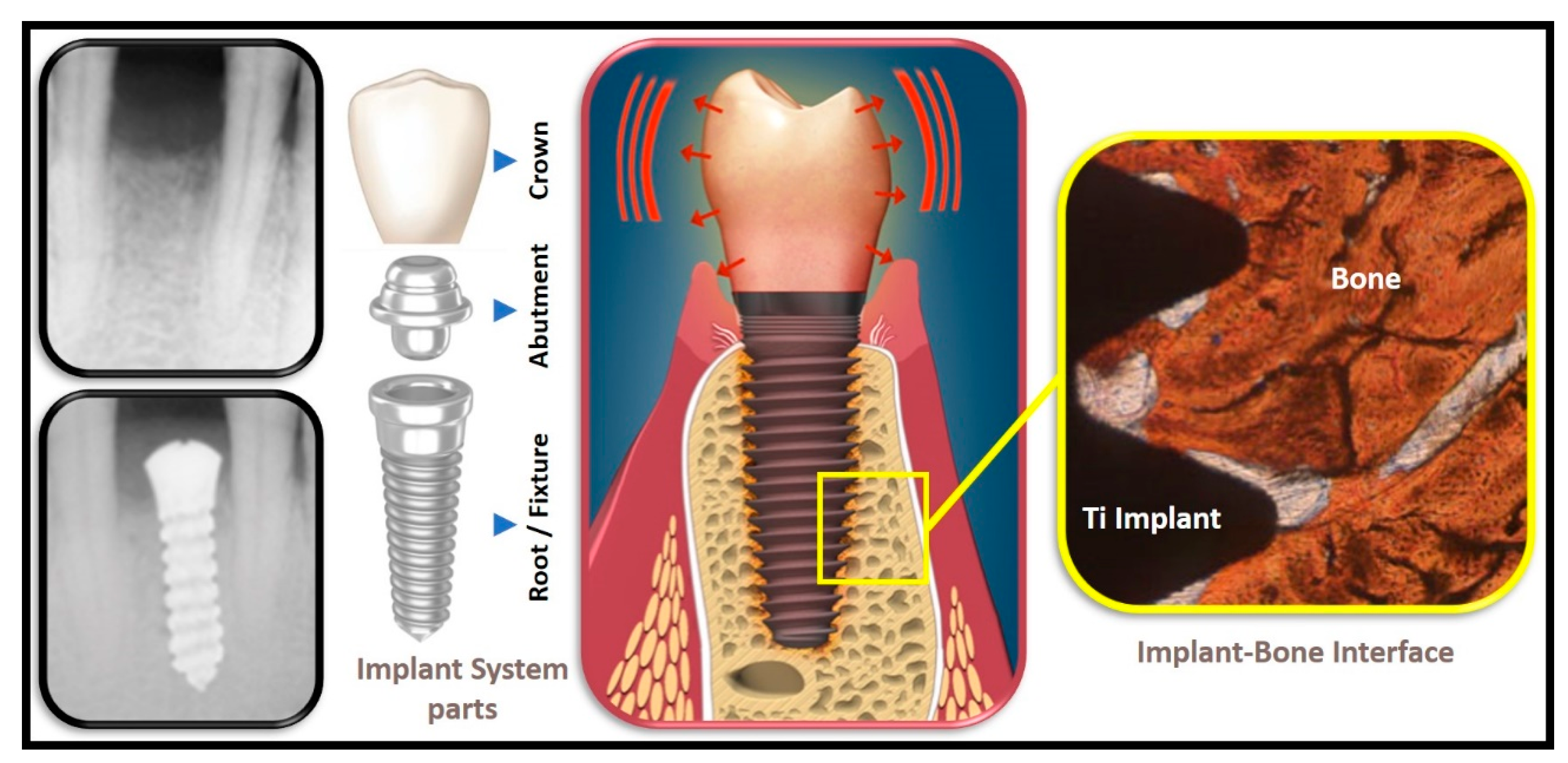
Figure 1. Oro-Dental Implantology, illustrating the main parts of a dental implant system and the implant–bone interface.
3. Conclusions
The cornerstone of successful dental implant therapy is osseointegration. Despite the fact that dental implants are a predictable (and preferred) treatment modality for the rehabilitation of partially and completely edentulous patients, high temperatures may cause irreversible damage to surrounding tissues and organs, with undesirable outcomes and sequels. The intrinsic time that characterizes the geometry of the dental implant has been introduced for the first time, and how the interplay between this “intrinsic” time and the exposure time of the thermal load influences temperature changes has been shown graphically. It is concluded that the exact analytical solution obtained here, despite its simplicity, encapsulates all the physics, and it nicely reproduces the key features previously obtained in other numerical analyses. Thermal stress should not be ignored in evaluating the performance of metal dental implants
[31][32][33][34,35,36]. It can benefit the dental implant manufacturer, dental diagnostic (accurate computed tomography scanning) industry, clinical operator, and the patient, consequently, via considering or controlling intra-oral temperatures and minimizing or preventing peri-implant tissue(s) damage and the onset of osteotomy-related side effects; this would therefore complement the overall treatment plan for an enhanced dental implant stability, free of the undesirable interference (osseointegration at the local cellular level) at the bone–implant interface, whether for immediate or delayed loading strategies (in various bone-types/conditions)
[31][32][33][34][34,35,36,37]. It is worth mentioning here to consider shorter dental implants (such as 4.0 × 4.0 mm) as an alternative to longer fixtures
[35][38].
ItWe is conclude
d that the exact analytical solution obtained here, despite its simplicity, encapsulates all the physics, and it nicely reproduces the key features previously obtained in other numerical analyses. Thermal stress should not be ignored in evaluating the performance of metal dental implants. This work can benefit the dental implant manufacturer, dental diagnostic (accurate computed tomography scanning) industry, clinical operator, and the patient, consequently, via considering or controlling intra-oral temperatures and minimizing or preventing peri-implant tissue(s) damage and the onset of osteotomy-related side effects; this would therefore complement the overall treatment plan for an enhanced dental implant stability, free of the undesirable interference (osseointegration at the local cellular level) at the one–implant interface, whether for immediate or delayed loading strategies (in various one-types/conditions).
ThePerspective: Our continuing work seeks to validate
theour results in a laboratory-based ex-vivo heat distribution model (in-House) employing osseointegrated human patient-grade Titanium dental implants that have been placed into porcine ribs (without coolant), followed y monitoring thermal changes (recorded and then plotted, quantifiably, under various conditions) using a CorDEX TP3R ToughPix DigiTherm Digital Thermal Camera, a currently-ongoing investigation
at our BioMAT’X (HAIDAR Lab).
@MDPIOpenAccess