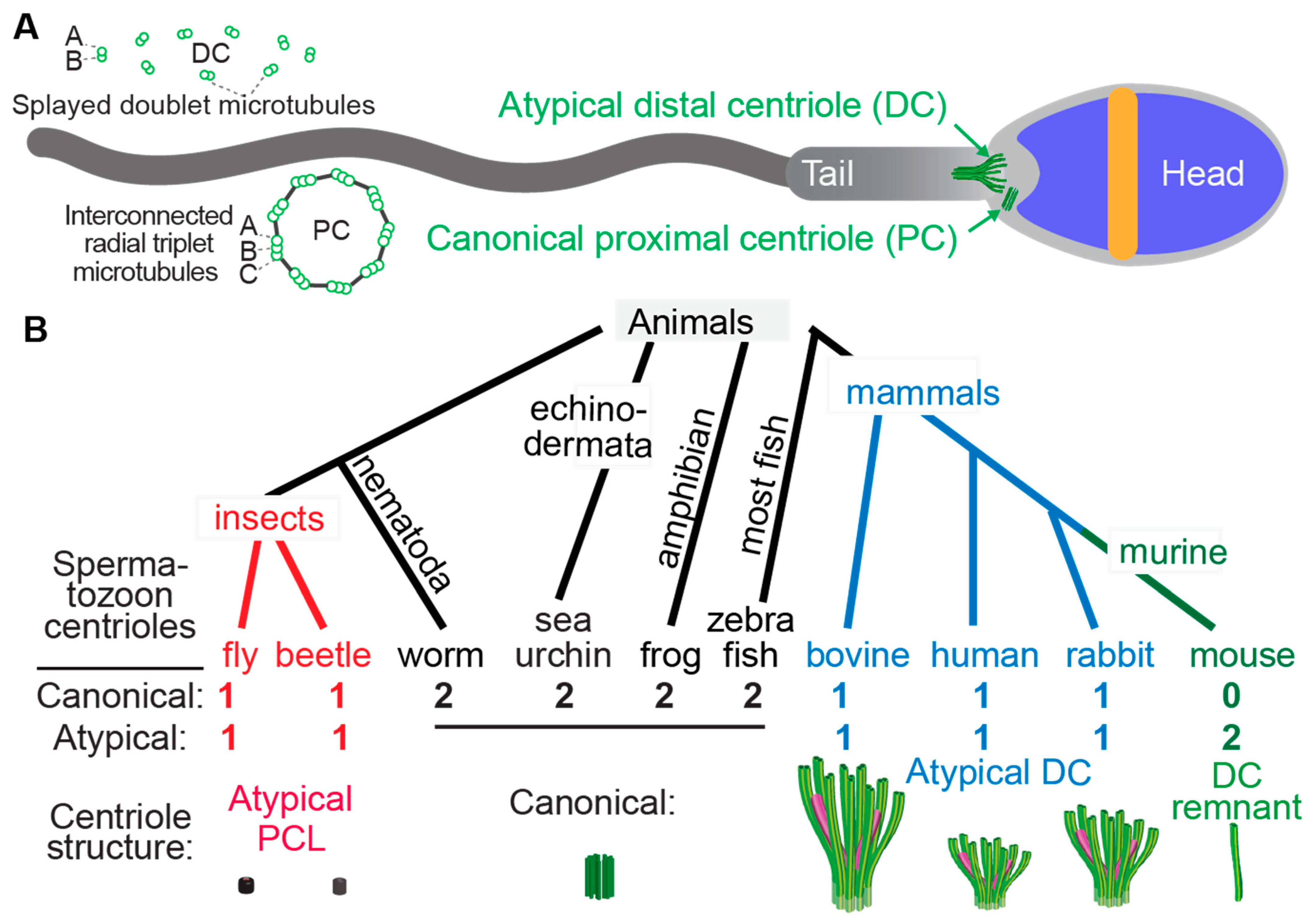
ThiInternal s manuscript addresses whether the sperm perm structures, the centriole evolved as part of Geoff A. Parker'shas an ancient and evolutionary sex cascade theory. Its analysis of fish literature proposes a novel hypothesis thatily conserved canonical structure with signature 9-fold, radially symmetric microtubules that form the cell’s centrosomes, cilia, and flagella. Most animal sperm atozoa have two centriole evolved an atypical composition in internal fertilizing species. This finding is consistent with the evolutionary sex cascade theory and provides one of few examples of sperm sub-cellular adaptions, one of which forms the spermatozoan flagellum. Both are delivered to the egg and constitute the embryo’s first two centrosomes. The spermatozoa of mammals and insects only have one recognizable centriole with a canonical structure.
- centriole
- sperm
- internal fertilization
- external fertilization
- sperm competition
- evolution
1. Introduction
2. Most Fish Species Studied Ultrastructurally Are External Fertilizers
3. Two Studied Externally Fertilizing Fish Species have Atypical Centriolar Composition
4. A High Rate of Internally Fertilizing Fish Species Studied Have Atypical Centriolar Composition
5. Species of the Internally Fertilizing Fish Subfamily Poeciliinae Have Atypical Centrioles
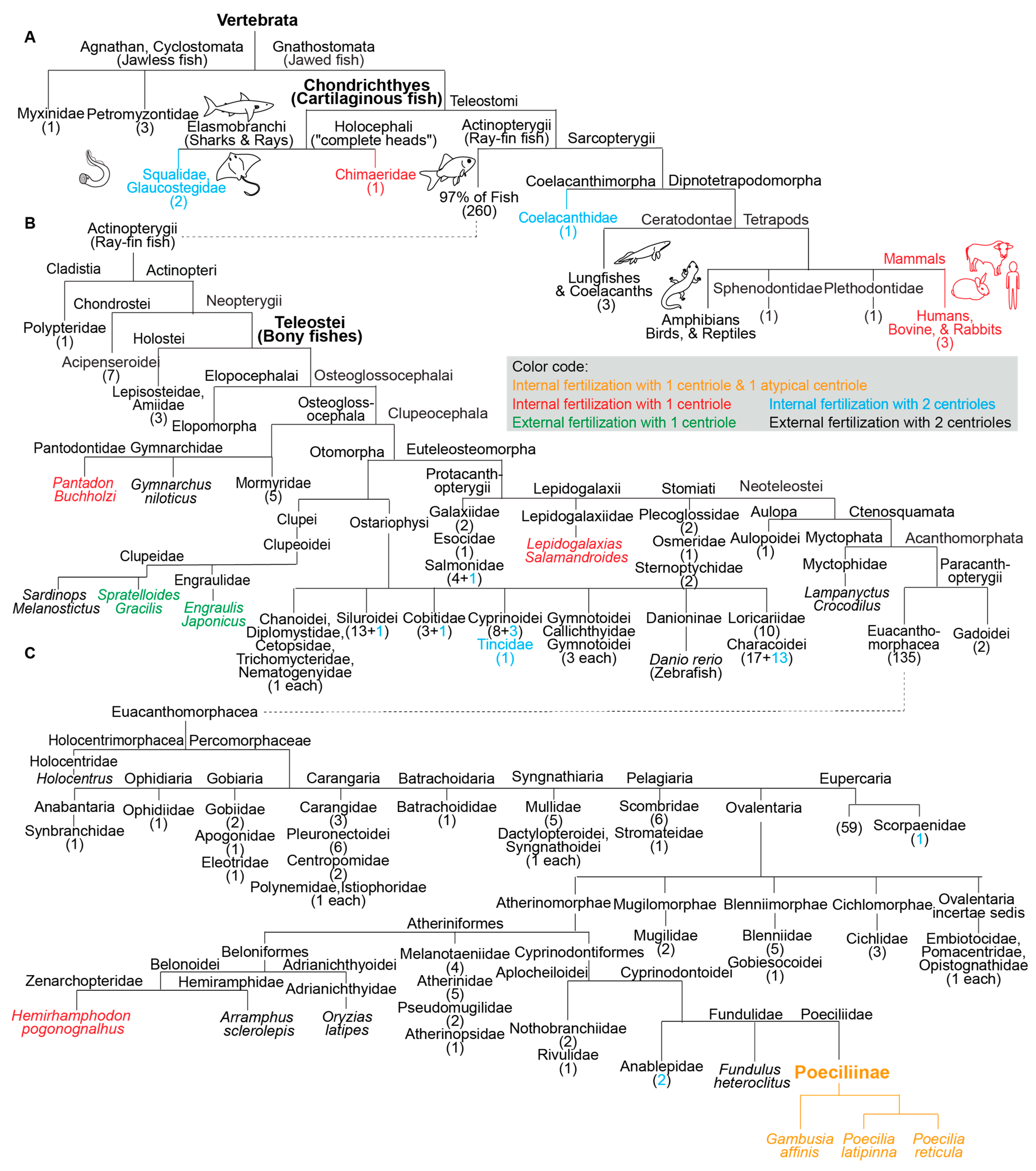
6. Species with a Single Canonical Centriole Evolved Independently Multiple Times
7. The Atypical Centriole Forms during Spermiogenesis
References
- Avidor-Reiss, T. Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function. Cells 2018, 7, 67.
- Allen, R.D. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J. Cell Biol. 1969, 40, 716–733.
- Vorobjev, I.A.; Chentsov Yu, S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982, 93, 938–949.
- Fawcett, D.W. A comparative view of sperm ultrastructure. Biol. Reprod. 1970, 2 (Suppl. S2), 90–127.
- Callaini, G.; Whitfield, W.G.; Riparbelli, M.G. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp. Cell Res. 1997, 234, 183–190.
- Uzbekov, R.E.; Avidor-Reiss, T. Principal Postulates of Centrosomal Biology. Version 2020. Cells 2020, 9, 2156.
- Woolley, D.M.; Fawcett, D.W. The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anat. Rec. 1973, 177, 289–301.
- Chakraborty, J. Neck region of gerbil spermatozoa. Gamete Res. 1979, 2, 25–34.
- Manandhar, G.; Sutovsky, P.; Joshi, H.C.; Stearns, T.; Schatten, G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998, 203, 424–434.
- Avidor-Reiss, T.; Fishman, E.L. It takes two (centrioles) to tango. Reproduction 2019, 157, 33–51.
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210.
- Khire, A.; Jo, K.H.; Kong, D.; Akhshi, T.; Blachon, S.; Cekic, A.R.; Hynek, S.; Ha, A.; Loncarek, J.; Mennella, V. Centriole remodeling during spermiogenesis in Drosophila. Curr. Biol. 2016, 26, 3183–3189.
- Fishman, E.L.; Jo, K.; Ha, A.; Royfman, R.; Zinn, A.; Krishnamurthy, M.; Avidor-Reiss, T. Atypical centrioles are present in Tribolium sperm. Open Biol. 2017, 7, 160334.
- Blachon, S.; Khire, A.; Avidor-Reiss, T. The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 2014, 197, 199–205.
- Dallai, R.; Mercati, D.; Lino-Neto, J.; Dias, G.; Lupetti, P. Evidence of a procentriole during spermiogenesis in the coccinellid insect Adalia decempunctata (L): An ultrastructural study. Arthropod. Struct. Dev. 2017, 46, 815–823.
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton 2015, 72, 576–584.
- Blachon, S.; Cai, X.; Roberts, K.A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 2009, 182, 133–144.
- Khanal, S.; Leung, M.R.; Royfman, A.; Fishman, E.L.; Saltzman, B.; Bloomfield-Gadelha, H.; Zeev-Ben-Mordehai, T.; Avidor-Reiss, T. A dynamic basal complex modulates mammalian sperm movement. Nat. Commun. 2021, 12, 3808.
- Leung, M.R.; Roelofs, M.C.; Ravi, R.T.; Maitan, P.; Henning, H.; Zhang, M.; Bromfield, E.G.; Howes, S.C.; Gadella, B.M.; Bloomfield-Gadelha, H.; et al. The multi-scale architecture of mammalian sperm flagella and implications for ciliary motility. EMBO J. 2021, 40, 107410.
- Manandhar, G.; Simerly, C.; Schatten, G. Centrosome reduction during mammalian spermiogenesis. Curr. Top. Dev. Biol. 2000, 49, 343–363.
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevicius, J.; Lukinavicius, G.; Elder, K.; et al. Parental genome unification is highly error-prone in mammalian embryos. Cell 2021, 184, 2860–2877.e22.
- Schneider, I.; de Ruijter-Villani, M.; Hossain, M.J.; Stout, T.A.E.; Ellenberg, J. Dual spindles assemble in bovine zygotes despite the presence of paternal centrosomes. J. Cell Biol. 2021, 220, e202010106.
- Kai, Y.; Kawano, H.; Yamashita, N. First mitotic spindle formation is led by sperm centrosome-dependent MTOCs in humans. Reproduction 2021, 161, 19–22.
- Amargant, F.; Pujol, A.; Ferrer-Vaquer, A.; Durban, M.; Martinez, M.; Vassena, R.; Vernos, I. The human sperm basal body is a complex centrosome important for embryo preimplantation development. Mol. Hum. Reprod. 2021, 27, gaab062.
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell Endocrinol. 2020, 518, 110987.
- Bobinnec, Y.; Khodjakov, A.; Mir, L.M.; Rieder, C.L.; Edde, B.; Bornens, M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998, 143, 1575–1589.
- Marshall, W.F. Centrioles take center stage. Curr. Biol. 2001, 11, 487–496.
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. From centriole biogenesis to cellular function: Centrioles are essential for cell division at critical developmental stages. Cell Cycle 2008, 7, 11–16.
- Tung, C.K.; Suarez, S.S. Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells 2021, 10, 1297.
- Higginson, D.M.; Miller, K.B.; Segraves, K.A.; Pitnick, S. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl. Acad. Sci. USA 2012, 109, 4538–4543.
- Druart, X. Sperm interaction with the female reproductive tract. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 348–352.
- Baccetti, B. The evolution of the sperm tail. Symp. Soc. Exp. Biol. 1982, 35, 521–532.
- Pitnick, S.; Wolfner, M.F.; Dorus, S. Post-ejaculatory modifications to sperm (PEMS). Biol Rev. Camb. Philos. Soc. 2020, 95, 365–392.
- Devigili, A.; Fitzpatrick, J.L.; Gasparini, C.; Ramnarine, I.W.; Pilastro, A.; Evans, J.P. Possible glimpses into early speciation: The effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. J. Evol. Biol. 2018, 31, 66–74.
- Jamieson, B.G. Avian spermatozoa: Structure and phylogeny. Reprod. Biol. Phylogeny Birds 2007, 6, 349–511.
- Hamilton, D.W.; Fawcett, D.W. Unusual features of the neck and middle-piece of snake spermatozoa. J. Ultrastruct. Res. 1968, 23, 81–97.
- Hess, R.A.; Thurston, R.J.; Gist, D.H. Ultrastructure of the turtle spermatozoon. Anat. Rec. 1991, 229, 473–481.
- Jamieson, B.G. Fish Evolution and Systematics: Evidence from Spermatozoa: With a Survey of Lophophorate, Echinoderm and Protochordate Sperm and an Account of Gamete Cryopreservation; Cambridge University Press: Cambridge, UK, 1991.
- Jamieson, B.G. Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes): Phylogeny, Reproductive System, Viviparity, Spermatozoa; CRC Press: Florida, FLA, USA, 2009.
- Froese, R. FishBase. World Wide Web Electronic Publication. 2022. Available online: http://www.fishbase.org (accessed on 1 January 2022).
- Gwo, J.C.; Lin, C.Y.; Yang, W.L.; Chou, Y.C. Ultrastructure of the sperm of blue sprat, Spratelloides gracilis; Teleostei, Clupeiformes, Clupeidae. Tissue Cell 2006, 38, 285–291.
- Mogi, K.; Misawa, K.; Utsunomiya, K.; Kawada, Y.; Yamazaki, T.; Takeuchi, S.; Toyoizumi, R. Optic chiasm in the species of order Clupeiformes, family Clupeidae: Optic chiasm of Spratelloides gracilis shows an opposite laterality to that of Etrumeus teres. Laterality 2009, 14, 495–514.
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes, 1st ed.; Natural History Press: New York, NY, USA, 1966.
- Fu, S.Y.; Jiang, J.H.; Yang, W.X.; Zhu, J.Q. A histological study of testis development and ultrastructural features of spermatogenesis in cultured Acrossocheilus fasciatus. Tissue Cell 2016, 48, 49–62.
- LEHODEY, P.; CHAI, F.; HAMPTON, J. FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae, 1988. Fish. Oceanogr. 2003, 12, 483–494.
- Fitzpatrick, J.L. Sperm competition and fertilization mode in fishes. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2020, 375, 20200074.
- Pavlov, D.; Emel’yanova, N. Comparative analysis of spermatozoa morphology in three fish species from the suborder Scorpaenoidei. J. Ichthyol. 2018, 58, 226–238.
- Rupik, W.; Huszno, J.; Klag, J. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)—Structural and ultrastructural studies. Micron 2011, 42, 833–839.
- Gwo, J.-C.; Ohta, H.; Okuzawa, K.; Wu, H.-C. Cryopreservation of sperm from the endangered Formosan landlocked salmon (Oncorhynchus masou formosanus). Theriogenology 1999, 51, 569–582.
- Gwo, J.-C.; Lin, X.; Gwo, H.; Wu, H.; Lin, P. The ultrastructure of Formosan landlocked salmon, Oncorbynchus masout formosanus, spermatozoon (Teleostei, Salmoniformes, Salmonidae). J. Submicrosc. Cytol. Pathol. 1996, 28, 33–40.
- Markova, M.D.; Zhivkova, R.S. Possible cytoskeletal structures of rainbow trout sperm revealed by electron microscopic observation after detergent extraction. Anim. Reprod. Sci. 2003, 79, 127–132.
- Guo, W.; Shao, J.; Li, P.; Wu, J.; Wei, Q. Morphology and ultrastructure of Brachymystax lenok tsinlingensis spermatozoa by scanning and transmission electron microscopy. Tissue Cell 2016, 48, 321–327.
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87.
- Thibault, R.E.; Schultz, R.J. Reproductive Adaptations among Viviparous Fishes (Cyprinodontiformes Poeciliidae). Evolution 1978, 32, 320–333.
- Billard, R.; Escaffre, A.-M.; Tramasaygues, N. La spermatogenèse de Poecilia reticulata. IV.—La spermiogenèse. Etude ul-trastructurale. Ann. Biol. Anim. Biochim. Biophys. 1970, 10, 493–510.
- Mattei, X.; Boisson, C. Le complexe centriolaire du spermatozoïde de Lebistes reticulatus. Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences Serie D 1966, 262, 2620.
- Balon, E.K. Epigenesis of an epigeneticist: The development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol. Rev. 1990, 1, 1–48.
- Grier, H.J. Ultrastructure of the testis in the teleost Poecilia latipinna. Spermiogenesis with reference to the intercentriolar lamellated body. J. Ultrastruct. Res. 1973, 45, 82–92.
- Wischnath, L. Atlas of Livebearers of the World; TFH publications: Neptune City, NJ, USA, 1993.
- Emel’yanova, N.G.; Pavlov, D.A. Gamete ultrastructure in some species of the family Mullidae from the South China Sea. J. Ichthyol. 2012, 52, 639–645.
- Seale, A. The Mosquito Fish, Gambusia affinis (Baird and Girard), in the Philippine Islands. Philipp. J. Sci. 1917, 12, 177–189.
- Hrbek, T.; Seckinger, J.; Meyer, A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol. Phylogenet. Evol. 2007, 43, 986–998.
- Stanley, H.P. The fine structure of spermatozoa of Hydrolagus colliei (Chondrichthyes, Holocephali). J. Ultrastruct. Res. 1983, 83, 184–194.
- Armstrong, R.H. Alaska’s Fish: A Guide to Selected Species; Alaska Northwest Books: Portland, OR, USA, 1996.
- Leung, L.K. Ultrastructure of the spermatozoon of Lepidogalaxias salamandroides and its phylogenetic significance. Gamete Res. 1988, 19, 41–49.
- Allen, G.R.; Midgley, S.H.; Allen, M. Freshwater Fishes of Australia; Western Australian Museum: Perth, Australia, 1989.
- Jamieson, B.G. Complex spermatozoon of the live-bearing half-beak, Hemirhamphodon pogonognathus (Bleeker): Ultrastructural description (Euteleostei, Atherinomorpha, Beloniformes). Gamete Res. 1989, 24, 247–259.
- Baensch, H.; Riehl, R. Aquarien Atlas; Mergus: Melle, Germany, 1987.
- Longo, F.J.; Anderson, E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J. Cell Biol. 1968, 39, 339–368.
- Grier, H.J. Sperm development in the teleost Oryzias latipes. Cell Tissue Res. 1976, 168, 419–431.
- Avidor-Reiss, T.; Turner, K. The Evolution of Centriole Structure: Heterochrony, Neoteny, and Hypermorphosis. Results Probl. Cell Differ. 2019, 67, 3–15.
- Dallai, R.; Mercati, D.; Bu, Y.; Yin, Y.W.; Callaini, G.; Riparbelli, M.G. The spermatogenesis and sperm structure of Acerentomon microrhinus (Protura, Hexapoda) with considerations on the phylogenetic position of the taxon. Zoomorphology 2010, 129, 61–80.
