Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 3 by Amina Yu.
Internal sperm structures, the centriole has an ancient and evolutionarily conserved canonical structure with signature 9-fold, radially symmetric microtubules that form the cell’s centrosomes, cilia, and flagella. Most animal spermatozoa have two centrioles, one of which forms the spermatozoan flagellum. Both are delivered to the egg and constitute the embryo’s first two centrosomes. The spermatozoa of mammals and insects only have one recognizable centriole with a canonical structure.
- centriole
- sperm
- internal fertilization
- external fertilization
- sperm competition
- evolution
1. Introduction
Little is known about the structural evolution of the sperm neck and the centrioles within it (Figure 1).
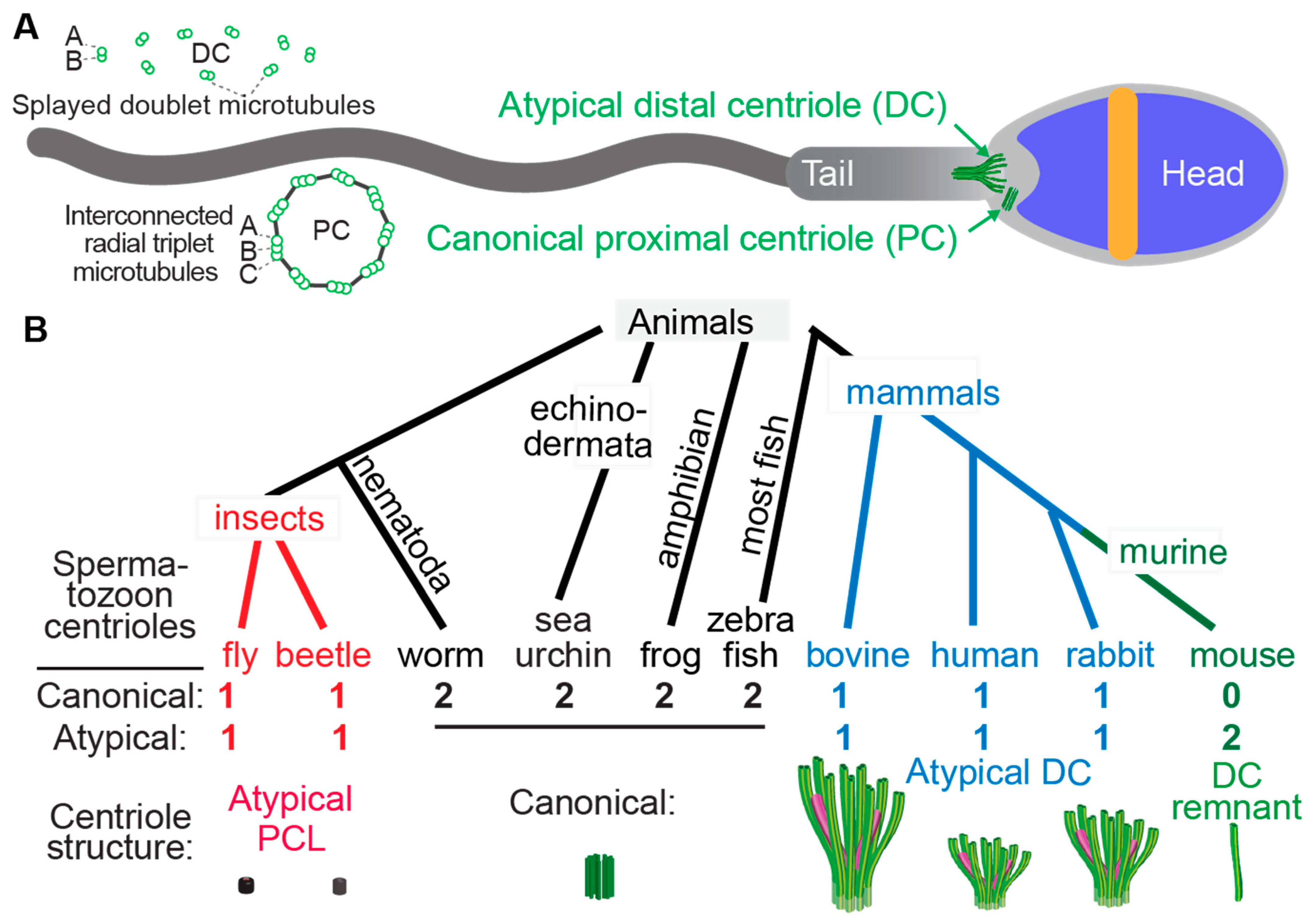
Figure 1. Atypical centrioles have distinct structures. (A) A mammalian sperm showing the locations of the atypical and canonical centrioles in the sperm neck. To the left, a cross section of the PC and DC depicting microtubule organization. (B) The number and size of spermatozoan centrioles vary throughout evolution. Shown are four animal groups (color-coded) organized based on the number and type of their spermatozoan centrioles. Animals with two centrioles (black). Animals with just one canonical centriole also have an atypical centriole: the proximal centriole-like (PCL, red) structure in insects and the atypical distal centriole (DC, blue) in mammals. Animals with DC remanent (green). Color code in the Atypical DC: green color marks the presence of microtubules, and magenta marks rod proteins.
Centrioles have a distinct structure that has been maintained since the last eukaryotic common ancestor (LECA) about 1.5 billion years ago (Hodges et al., 2010). This structure is easily identifiable by the microtubule bundles organized into nine-fold symmetry, as observed by electron microscopy (Figure 1) [1][2][3][4]. While the most common and ancestral form consists of nine triplet microtubules, centrioles with nine doublet or singlet microtubules are also known to occur [5][6]. Based on these structural criteria, it was observed that many animal cell types including sperm cells have two centrioles, which is the ancestral centriolar composition of a sperm cell (Figure 1B). However, some exceptions were noted to have atypical centriolar composition. For example, both insect and mammalian sperm cells either have only one or no centrioles as defined by the above structural criteria [7][8][9][10][11][12][13]. Only recently, due to the discovery of the protein composition of the centriole, a second functional though structurally atypical centriole was observed first in insects [13][14][15][16][17] and later in mammals [11][18][19]. Suggesting that animal sperm cells indeed have two centrioles when they were thought to possess only one typical sperm centriole, even if they cannot be identified using classical structural criteria.
The two centrioles in animal sperm maintain distinct functions and locations relative to the nucleus. The centriole near the nucleus is called the proximal centriole, while the more distant centriole, which also nucleates the flagellum, is called the distal centriole [20]. Both centrioles form a centrosome post-fertilization in the zygote [10][21][22][23][24][25].
Centrioles are involved in several functions in somatic cells, particularly accurate cell division, ciliogenesis, and the synthesis of new centrioles [26][27][28][29]. However, in sperm, their main purpose is to form the tail (for example, axoneme). Additionally, they have been proposed to regulate tail movement [18]. Consequently, it could be predicted that centrioles become more specialized (for example, atypical) according to the environment that the sperm is moving through to maximize efficiency. For example, in sperm movement through water, which is the condition in external fertilization, centrioles may remain typical because water is a simple and homogenous environment, and sperm must swim for only a short time directly toward the eggs. However, when sperm travel through the female reproductive tract such as in internal fertilization, they may traverse more complex environments, triggering the next step in the sexual cascade [30][31]. Indeed, sperm swimming through water is a simple process relative to the process of internal fertilization [32].
The female reproductive tract is comprised of several environments that create distinct barriers for sperm travel or storage and may present more opportunities for sperm competition. In mammals, these barriers include the cervix, uterotubal junction (UTJ), and oviduct [33]. In insects, they include storage in specialized organs [34]. Additionally, the female reproductive tract has viscous mucus through which the sperm must move to reach the egg [35]. These properties of the female reproductive tract generate evolutionary pressure on sperm movement. TWe, therefore, it was hypothesized that internal fertilization in an elaborate female reproductive tract benefits from remodeled centrioles, whereas external fertilization in a simple water environment benefits from canonical centrioles.
During mammalian evolution, internal fertilization existed before the sperm centriole became atypical. Mammals were preceded by Synapsids, and most Synapsid groups (including birds [36], snakes [37], and turtles [38]) have ancestral centriolar composition with two canonical centrioles. Therefore, internal fertilization preceded the appearance of atypical centrioles in mammalian evolution.
Fish are among the most ancient vertebrate animals, are characterized by incredible diversity, and have a range of reproductive modes including internal and external fertilization. Fish sperm is commonly studied in decisions on the phylogenetic relationships of distinct species, and fish have a variety of sperm morphologies and structures [39][40]. It was noted that the sperm centrioles, like other sperm structures, could take different forms in fish. However, the relationship between centriolar structure and reproduction strategy is unclear. it was determined that the association of sperm centriolar composition with internal or external fertilization strategies. It was found a strong and statistically significant correlation between internal fertilization and atypical centriolar composition, suggesting that atypical centrioles evolved as part of the evolutionary sexual cascade that occurred after the appearance of internal fertilization.
2. Most Fish Species Studied Ultrastructurally Are External Fertilizers
It was described that electron microscopy studies of sperm, it was surveyed reports on the sperm centrioles of 277 fish species . This is a small fraction of the at least 34,000 extant species. Most species (or their close relatives) were external fertilizers (87.7%, 243/277), and few were internal fertilizers (12.3%, 34/277), a ratio of 7.1 to 1. This ratio is lower than estimated in nature, as it has been reported that more than 95% of fish species (ratio of more than 19:1) are external fertilizers [41], indicating a bias toward ultrastructural studies of internally fertilizing fish.
Of the 277 species studied, 268 (96.8%, 268/277) were reported to have two canonical centrioles, while nine species (3.2%, 9/277) were reported to have only one canonical centriole. This is a ratio of 30 to 1, indicating that most fish studied were characterized by ancestral centriolar composition.
3. Two Studied Externally Fertilizing Fish Species have Atypical Centriolar Composition
Of the 243 studied species that reproduce by external fertilization, two were reported to have one canonical centriole, representing 0.8% of the external fertilizers. These species reports are as follows:
Gwo et al. reported on p.286 that in Spratelloides gracilis, “No proximal centriole was identified” [42], while it was reported that this species reproduces by external fertilization [43][44].
Fu et al. reported on p.60 that “the proximal centriole of Engraulis japonicus (Engraulididae) is indistinct” [45], and it was also reported to be an external fertilizer [46][47].
Many other externally fertilizing fish have two canonical centrioles. This includes fish from the families Scorpaenidae and Danioninae (which includes the important model species Zebrafish) [48][49]. For example, papers on five species of Salmonidae was studied, all of which have two canonical centrioles [50][51][52][53][54].
4. A High Rate of Internally Fertilizing Fish Species Studied Have Atypical Centriolar Composition
Of the 34 species that reproduce by internal fertilization, seven (20.6%) were reported to have a single sperm centriole. The Z test was used for two population proportions and found that internally fertilizing fish had a statistically significant higher proportion of sperm with a single canonical centriole than externally fertilizing fish (p < 0.00001). The relative rate reduction was 19.8% for the reported single canonical centriole. The odds ratio of 32.4 for the group reported with one canonical centriole over the group reported with two canonical centrioles indicates a high difference. A similar conclusion can be drawn if the data are calculated based on the number of genera studied instead of species name. This finding suggests that the evolutionary sexual cascade of events resulted first in internal fertilization and later, in some species, in atypical centriolar composition.
5. Species of the Internally Fertilizing Fish Subfamily Poeciliinae Have Atypical Centrioles
Of all the fish analyzed, one group appears to have atypical centrioles: the subfamily Poeciliinae (belonging to Teleostei, or bony fishes with protrusible jaws) (Figure 2). Species of the subfamily Poeciliinae and the family Poeciliidae are internal fertilizers [55].
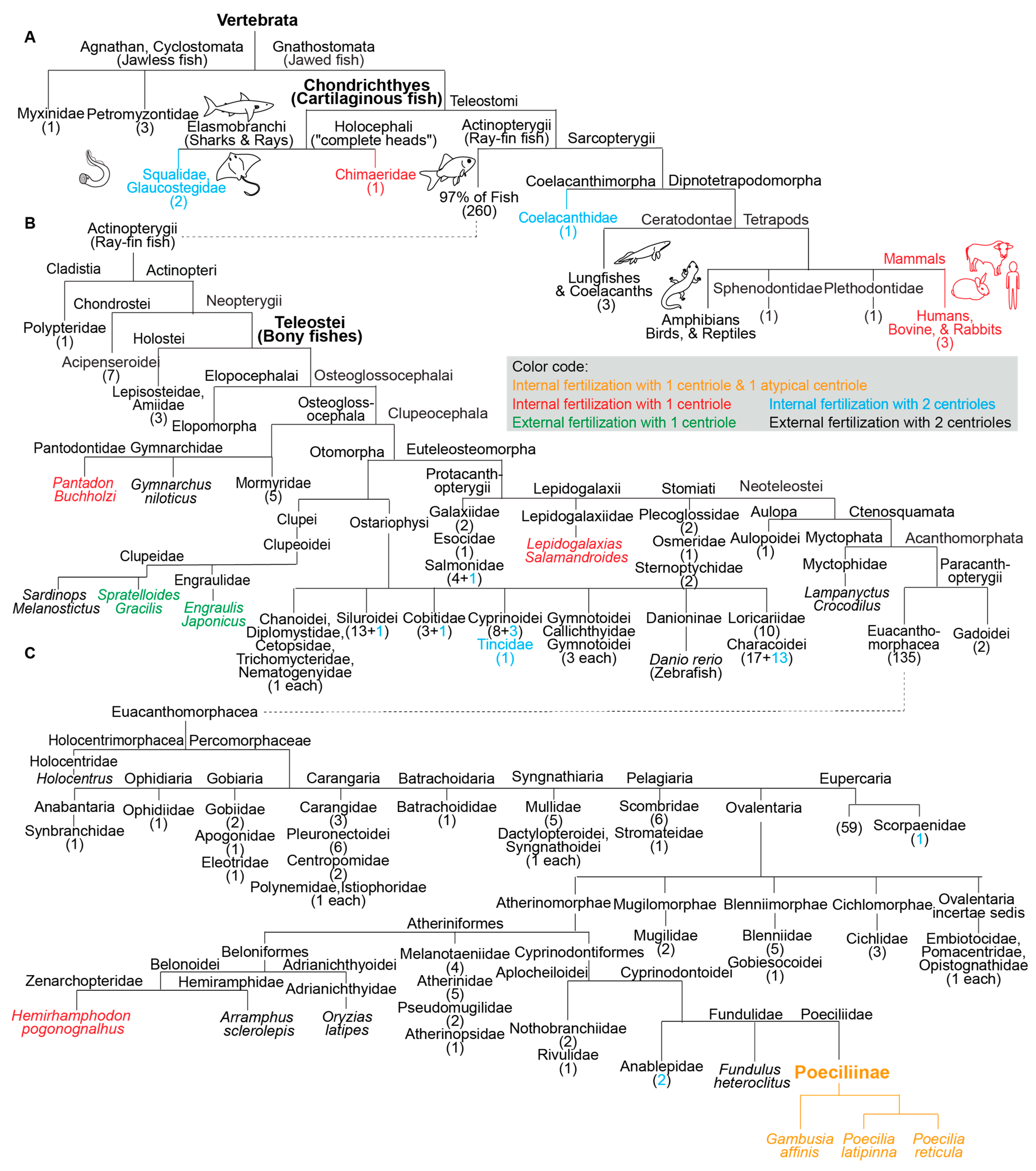

Figure 2. Fish species with a single canonical centriole evolved independently multiple times from an ancestral state with two canonical centrioles. (A–C) A phylogenetic tree depicting fertilization mode (internal or external) and canonical centriole number (1 or 2) in the main branches of vertebrates and fish. (A) Depicts the main fish groups. (B) Depicts ray-fin fish (Actinopterygii). (C) Depicts Euacanthomorphacea. In some cases, it was provided that the scientific name and common name in parentheses. Vertebrata, Chondrichthyes (Cartilaginous fish), Teleostei (Bony fishes), and Poeciliinae are bolded and enlarged because they are specifically referenced in the text. The number of fish species in a category appear as a numeral in parentheses. The figure contains the names of all fish families analyzed in this survey.
Jamieson reported on p.470 for Poecilia reticulata that “proximal centriole triplets become occluded and is reduced to a remnant by maturity” [39][40][56][57]. This species is an internal fertilizer [58].
Grier et al. reported on p.86 for Poecilia latipinna that “a rather indistinct electron dense structure was present in the sperm neck” [59]. This species is an internal fertilizer [60].
Emel’yanova and Pavlov reported on p.97 that for Gambusia affinis, the “proximal centriole (pc) is electron dense and no longer contains distinct tubules” [61]. This species is an internal fertilizer [62].
The subfamily Poeciliinae includes many genera in addition to Poecilia and Gambusia that are thought to belong to distinct tribes [63]. Therefore, it is possible that the subfamily Poeciliinae is a monophyletic group that ancestrally had an atypical centriole.
6. Species with a Single Canonical Centriole Evolved Independently Multiple Times
In addition to the three studied species of the subfamily Poeciliinae, it was identified four additional species with a single canonical spermatozoan centriole that are also internal fertilizers; each of them occupies a distinct phylogenetic clade (Figure 1). The reported internal fertilizers included one Chondrichthyes species (cartilaginous fishes) and three species from distantly related Teleostei (bony fishes with protrusible jaws) groups.
Hydrolagus colliei (Chondrichthyes): It was reported on p.193 that “the proximal centriole has not been seen in mature Hydrolagus sperm” [64], and this species was reported to be an internal fertilizer [65].
Lepidogalaxias salamandroides: It was reported on p.42 by Leung that “No typical 9 × 3 microtubule centrioles have been found in the spermatozoon, but a basal body was located at the anterior of the axoneme” [66], and this species was reported to be an internal fertilizer [67]. Jamieson reported on p.156 for Lepidogalaxias salamandroides that “no typical triplet centrioles have been observed. A basal body…located at the anterior end of the flagellum consists of 9 doublets” [39].
Hemirhamphodon pogonognathus: It was reported on p.251 that “A proximal centriole has not been identified” [68] and this species was reported to be an internal fertilizer [69].
Pantodon buchholzi: It was reported on p.118 that “the centriolar complex consists of a single centriole (basal body), although an additional centriole, presumably the proximal centriole, is sometimes observed parallel to it” [39]. This species was reported to be an internal fertilizer [39][65].
Additionally, the two reported external fertilizers were from two unrelated Teleostei groups. Spratelloides gracilis (silver-stripe round herring) and Sardinops melanostictus both belong to the Clupeoidei suborder, though they are from distinct families (Engraulidae or Anchovies and Clupeidae). The Clupeidae family includes the species Sardinops melanostictus, whose spermatozoa have two canonical centrioles [39]. Therefore, the appearance of atypical centriolar composition in the Clupeoidei suborder occurred twice independently.
The nine species with a single canonical centriole belonged to clades that include many more species with two canonical centrioles, suggesting that ancestrally, they had spermatozoa with two canonical centrioles (Figure 2). Therefore, it appears that a single canonical centriole is a recent innovation in the evolution of these fish species, and that a change from ancestral centriolar composition to atypical centriolar composition took place independently multiple times, indicating convergent evolution due to positive selection.
7. The Atypical Centriole Forms during Spermiogenesis
In many animal species, the canonical structures of the proximal and distal centrioles are maintained during spermiogenesis (the differentiation of a round spermatid to a spermatozoon in the testes) [1][70]. Similarly, the canonical structures of the proximal and distal centrioles are maintained during spermatogenesis in fish with ancestral centriolar composition [71]. In mammals and insects, the atypical centriole forms during spermiogenesis via distinct mechanisms [10][12][13][72]. In insects, an atypical proximal centriole forms during spermiogenesis as a new centriole. In mammals, the canonical distal centriole found in the early spermatid is remodeled during spermiogenesis to become atypical. This indicates that insects and mammals use very different mechanisms to create the atypical centrioles during spermiogenesis.
Spermiogenesis in the fish Gambusia affinis, which has atypical centrioles, was studied [59]. In this species, the canonical proximal and distal centrioles are present in the early spermatid differentiation stage, but the proximal centriole becomes occluded in the later spermatid stage, and its microtubules disappear by the end of spermiogenesis. This situation is like that of insects, in that their atypical sperm centriole is the PC, not the DC, as is the case in mammals. However, Gambusia affinis is like mammals in that its atypical sperm centriole is remodeled from a spermatid centriole and not formed initially as an atypical centriole in the spermatid, as it is in insects. It therefore appears that atypical centriolar composition is achieved by distinct subcellular mechanisms in mammals, insects, and fish, which is consistent with the idea that they all evolved independently.
References
- Avidor-Reiss, T. Rapid Evolution of Sperm Produces Diverse Centriole Structures that Reveal the Most Rudimentary Structure Needed for Function. Cells 2018, 7, 67.
- Allen, R.D. The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J. Cell Biol. 1969, 40, 716–733.
- Vorobjev, I.A.; Chentsov Yu, S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 1982, 93, 938–949.
- Fawcett, D.W. A comparative view of sperm ultrastructure. Biol. Reprod. 1970, 2 (Suppl. S2), 90–127.
- Callaini, G.; Whitfield, W.G.; Riparbelli, M.G. Centriole and centrosome dynamics during the embryonic cell cycles that follow the formation of the cellular blastoderm in Drosophila. Exp. Cell Res. 1997, 234, 183–190.
- Uzbekov, R.E.; Avidor-Reiss, T. Principal Postulates of Centrosomal Biology. Version 2020. Cells 2020, 9, 2156.
- Woolley, D.M.; Fawcett, D.W. The degeneration and disappearance of the centrioles during the development of the rat spermatozoon. Anat. Rec. 1973, 177, 289–301.
- Chakraborty, J. Neck region of gerbil spermatozoa. Gamete Res. 1979, 2, 25–34.
- Manandhar, G.; Sutovsky, P.; Joshi, H.C.; Stearns, T.; Schatten, G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998, 203, 424–434.
- Avidor-Reiss, T.; Fishman, E.L. It takes two (centrioles) to tango. Reproduction 2019, 157, 33–51.
- Fishman, E.L.; Jo, K.; Nguyen, Q.P.H.; Kong, D.; Royfman, R.; Cekic, A.R.; Khanal, S.; Miller, A.L.; Simerly, C.; Schatten, G.; et al. A novel atypical sperm centriole is functional during human fertilization. Nat. Commun. 2018, 9, 2210.
- Khire, A.; Jo, K.H.; Kong, D.; Akhshi, T.; Blachon, S.; Cekic, A.R.; Hynek, S.; Ha, A.; Loncarek, J.; Mennella, V. Centriole remodeling during spermiogenesis in Drosophila. Curr. Biol. 2016, 26, 3183–3189.
- Fishman, E.L.; Jo, K.; Ha, A.; Royfman, R.; Zinn, A.; Krishnamurthy, M.; Avidor-Reiss, T. Atypical centrioles are present in Tribolium sperm. Open Biol. 2017, 7, 160334.
- Blachon, S.; Khire, A.; Avidor-Reiss, T. The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 2014, 197, 199–205.
- Dallai, R.; Mercati, D.; Lino-Neto, J.; Dias, G.; Lupetti, P. Evidence of a procentriole during spermiogenesis in the coccinellid insect Adalia decempunctata (L): An ultrastructural study. Arthropod. Struct. Dev. 2017, 46, 815–823.
- Gottardo, M.; Callaini, G.; Riparbelli, M.G. Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton 2015, 72, 576–584.
- Blachon, S.; Cai, X.; Roberts, K.A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics 2009, 182, 133–144.
- Khanal, S.; Leung, M.R.; Royfman, A.; Fishman, E.L.; Saltzman, B.; Bloomfield-Gadelha, H.; Zeev-Ben-Mordehai, T.; Avidor-Reiss, T. A dynamic basal complex modulates mammalian sperm movement. Nat. Commun. 2021, 12, 3808.
- Leung, M.R.; Roelofs, M.C.; Ravi, R.T.; Maitan, P.; Henning, H.; Zhang, M.; Bromfield, E.G.; Howes, S.C.; Gadella, B.M.; Bloomfield-Gadelha, H.; et al. The multi-scale architecture of mammalian sperm flagella and implications for ciliary motility. EMBO J. 2021, 40, 107410.
- Manandhar, G.; Simerly, C.; Schatten, G. Centrosome reduction during mammalian spermiogenesis. Curr. Top. Dev. Biol. 2000, 49, 343–363.
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevicius, J.; Lukinavicius, G.; Elder, K.; et al. Parental genome unification is highly error-prone in mammalian embryos. Cell 2021, 184, 2860–2877.e22.
- Schneider, I.; de Ruijter-Villani, M.; Hossain, M.J.; Stout, T.A.E.; Ellenberg, J. Dual spindles assemble in bovine zygotes despite the presence of paternal centrosomes. J. Cell Biol. 2021, 220, e202010106.
- Kai, Y.; Kawano, H.; Yamashita, N. First mitotic spindle formation is led by sperm centrosome-dependent MTOCs in humans. Reproduction 2021, 161, 19–22.
- Amargant, F.; Pujol, A.; Ferrer-Vaquer, A.; Durban, M.; Martinez, M.; Vassena, R.; Vernos, I. The human sperm basal body is a complex centrosome important for embryo preimplantation development. Mol. Hum. Reprod. 2021, 27, gaab062.
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell Endocrinol. 2020, 518, 110987.
- Bobinnec, Y.; Khodjakov, A.; Mir, L.M.; Rieder, C.L.; Edde, B.; Bornens, M. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 1998, 143, 1575–1589.
- Marshall, W.F. Centrioles take center stage. Curr. Biol. 2001, 11, 487–496.
- Rodrigues-Martins, A.; Riparbelli, M.; Callaini, G.; Glover, D.M.; Bettencourt-Dias, M. From centriole biogenesis to cellular function: Centrioles are essential for cell division at critical developmental stages. Cell Cycle 2008, 7, 11–16.
- Tung, C.K.; Suarez, S.S. Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization. Cells 2021, 10, 1297.
- Higginson, D.M.; Miller, K.B.; Segraves, K.A.; Pitnick, S. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl. Acad. Sci. USA 2012, 109, 4538–4543.
- Druart, X. Sperm interaction with the female reproductive tract. Reprod. Domest. Anim. 2012, 47 (Suppl. S4), 348–352.
- Baccetti, B. The evolution of the sperm tail. Symp. Soc. Exp. Biol. 1982, 35, 521–532.
- Pitnick, S.; Wolfner, M.F.; Dorus, S. Post-ejaculatory modifications to sperm (PEMS). Biol Rev. Camb. Philos. Soc. 2020, 95, 365–392.
- Devigili, A.; Fitzpatrick, J.L.; Gasparini, C.; Ramnarine, I.W.; Pilastro, A.; Evans, J.P. Possible glimpses into early speciation: The effect of ovarian fluid on sperm velocity accords with post-copulatory isolation between two guppy populations. J. Evol. Biol. 2018, 31, 66–74.
- Jamieson, B.G. Avian spermatozoa: Structure and phylogeny. Reprod. Biol. Phylogeny Birds 2007, 6, 349–511.
- Hamilton, D.W.; Fawcett, D.W. Unusual features of the neck and middle-piece of snake spermatozoa. J. Ultrastruct. Res. 1968, 23, 81–97.
- Hess, R.A.; Thurston, R.J.; Gist, D.H. Ultrastructure of the turtle spermatozoon. Anat. Rec. 1991, 229, 473–481.
- Jamieson, B.G. Fish Evolution and Systematics: Evidence from Spermatozoa: With a Survey of Lophophorate, Echinoderm and Protochordate Sperm and an Account of Gamete Cryopreservation; Cambridge University Press: Cambridge, UK, 1991.
- Jamieson, B.G. Reproductive Biology and Phylogeny of Fishes (Agnathans and Bony Fishes): Phylogeny, Reproductive System, Viviparity, Spermatozoa; CRC Press: Florida, FLA, USA, 2009.
- Froese, R. FishBase. World Wide Web Electronic Publication. 2022. Available online: http://www.fishbase.org (accessed on 1 January 2022).
- Gwo, J.C.; Lin, C.Y.; Yang, W.L.; Chou, Y.C. Ultrastructure of the sperm of blue sprat, Spratelloides gracilis; Teleostei, Clupeiformes, Clupeidae. Tissue Cell 2006, 38, 285–291.
- Mogi, K.; Misawa, K.; Utsunomiya, K.; Kawada, Y.; Yamazaki, T.; Takeuchi, S.; Toyoizumi, R. Optic chiasm in the species of order Clupeiformes, family Clupeidae: Optic chiasm of Spratelloides gracilis shows an opposite laterality to that of Etrumeus teres. Laterality 2009, 14, 495–514.
- Breder, C.M.; Rosen, D.E. Modes of Reproduction in Fishes, 1st ed.; Natural History Press: New York, NY, USA, 1966.
- Fu, S.Y.; Jiang, J.H.; Yang, W.X.; Zhu, J.Q. A histological study of testis development and ultrastructural features of spermatogenesis in cultured Acrossocheilus fasciatus. Tissue Cell 2016, 48, 49–62.
- LEHODEY, P.; CHAI, F.; HAMPTON, J. FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae FAO species catalogue, Vol. 7. Clupeoid fishes of the world (Suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings. Part 2-Engraulididae, 1988. Fish. Oceanogr. 2003, 12, 483–494.
- Fitzpatrick, J.L. Sperm competition and fertilization mode in fishes. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2020, 375, 20200074.
- Pavlov, D.; Emel’yanova, N. Comparative analysis of spermatozoa morphology in three fish species from the suborder Scorpaenoidei. J. Ichthyol. 2018, 58, 226–238.
- Rupik, W.; Huszno, J.; Klag, J. Cellular organisation of the mature testes and stages of spermiogenesis in Danio rerio (Cyprinidae; Teleostei)—Structural and ultrastructural studies. Micron 2011, 42, 833–839.
- Gwo, J.-C.; Ohta, H.; Okuzawa, K.; Wu, H.-C. Cryopreservation of sperm from the endangered Formosan landlocked salmon (Oncorhynchus masou formosanus). Theriogenology 1999, 51, 569–582.
- Gwo, J.-C.; Lin, X.; Gwo, H.; Wu, H.; Lin, P. The ultrastructure of Formosan landlocked salmon, Oncorbynchus masout formosanus, spermatozoon (Teleostei, Salmoniformes, Salmonidae). J. Submicrosc. Cytol. Pathol. 1996, 28, 33–40.
- Markova, M.D.; Zhivkova, R.S. Possible cytoskeletal structures of rainbow trout sperm revealed by electron microscopic observation after detergent extraction. Anim. Reprod. Sci. 2003, 79, 127–132.
- Guo, W.; Shao, J.; Li, P.; Wu, J.; Wei, Q. Morphology and ultrastructure of Brachymystax lenok tsinlingensis spermatozoa by scanning and transmission electron microscopy. Tissue Cell 2016, 48, 321–327.
- Figueroa, E.; Valdebenito, I.; Zepeda, A.B.; Figueroa, C.A.; Dumorné, K.; Castillo, R.L.; Farias, J.G. Effects of cryopreservation on mitochondria of fish spermatozoa. Rev. Aquac. 2017, 9, 76–87.
- Thibault, R.E.; Schultz, R.J. Reproductive Adaptations among Viviparous Fishes (Cyprinodontiformes Poeciliidae). Evolution 1978, 32, 320–333.
- Billard, R.; Escaffre, A.-M.; Tramasaygues, N. La spermatogenèse de Poecilia reticulata. IV.—La spermiogenèse. Etude ul-trastructurale. Ann. Biol. Anim. Biochim. Biophys. 1970, 10, 493–510.
- Mattei, X.; Boisson, C. Le complexe centriolaire du spermatozoïde de Lebistes reticulatus. Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences Serie D 1966, 262, 2620.
- Balon, E.K. Epigenesis of an epigeneticist: The development of some alternative concepts on the early ontogeny and evolution of fishes. Guelph Ichthyol. Rev. 1990, 1, 1–48.
- Grier, H.J. Ultrastructure of the testis in the teleost Poecilia latipinna. Spermiogenesis with reference to the intercentriolar lamellated body. J. Ultrastruct. Res. 1973, 45, 82–92.
- Wischnath, L. Atlas of Livebearers of the World; TFH publications: Neptune City, NJ, USA, 1993.
- Emel’yanova, N.G.; Pavlov, D.A. Gamete ultrastructure in some species of the family Mullidae from the South China Sea. J. Ichthyol. 2012, 52, 639–645.
- Seale, A. The Mosquito Fish, Gambusia affinis (Baird and Girard), in the Philippine Islands. Philipp. J. Sci. 1917, 12, 177–189.
- Hrbek, T.; Seckinger, J.; Meyer, A. A phylogenetic and biogeographic perspective on the evolution of poeciliid fishes. Mol. Phylogenet. Evol. 2007, 43, 986–998.
- Stanley, H.P. The fine structure of spermatozoa of Hydrolagus colliei (Chondrichthyes, Holocephali). J. Ultrastruct. Res. 1983, 83, 184–194.
- Armstrong, R.H. Alaska’s Fish: A Guide to Selected Species; Alaska Northwest Books: Portland, OR, USA, 1996.
- Leung, L.K. Ultrastructure of the spermatozoon of Lepidogalaxias salamandroides and its phylogenetic significance. Gamete Res. 1988, 19, 41–49.
- Allen, G.R.; Midgley, S.H.; Allen, M. Freshwater Fishes of Australia; Western Australian Museum: Perth, Australia, 1989.
- Jamieson, B.G. Complex spermatozoon of the live-bearing half-beak, Hemirhamphodon pogonognathus (Bleeker): Ultrastructural description (Euteleostei, Atherinomorpha, Beloniformes). Gamete Res. 1989, 24, 247–259.
- Baensch, H.; Riehl, R. Aquarien Atlas; Mergus: Melle, Germany, 1987.
- Longo, F.J.; Anderson, E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata. J. Cell Biol. 1968, 39, 339–368.
- Grier, H.J. Sperm development in the teleost Oryzias latipes. Cell Tissue Res. 1976, 168, 419–431.
- Avidor-Reiss, T.; Turner, K. The Evolution of Centriole Structure: Heterochrony, Neoteny, and Hypermorphosis. Results Probl. Cell Differ. 2019, 67, 3–15.
- Dallai, R.; Mercati, D.; Bu, Y.; Yin, Y.W.; Callaini, G.; Riparbelli, M.G. The spermatogenesis and sperm structure of Acerentomon microrhinus (Protura, Hexapoda) with considerations on the phylogenetic position of the taxon. Zoomorphology 2010, 129, 61–80.
More
