Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Jeevithan Elango.
Collagen, an extracellular protein, covers the entire human body and has several important biological functions in normal physiology. Collagen possibly binds with at least six different groups of receptors in biological cells. These are integrins, DDR, Glycoprotein VI, Osteoclast-associated receptor (OSCAR), LAIR-1, and uPARAP/Endo180.
- collagen
- receptors
- Cell signals
- Biological function
1. Integrins
Integrins are well-known cell surface heterodimeric receptors distributed to almost all biological cells, which regulate the development and pathological processes of organs. Integrins constitute a major group of receptors for extracellular matrix components, including collagens. Integrins are widely distributed in organs such as skin, kidney, lungs, muscles, inner ears, eyes, heart, blood vessels, vascular endothelium, heart, gut (Peyer’s patches), lymphatic endothelium, mesenchymal tissue, cartilage and periodontal ligaments; and cells such as macrophage, platelets, fibroblasts, immune cells, bone cells, cancer cells, leucocytes (all types), eosinophils, chondrocytes, embryonic stem cells and so on [15]. Integrins play an essential role in regulating cell signals, migration, survival and differentiation [13,16]. At present, four types of collagen-binding integrins have been identified, namely alpha 1 beta 1 (α1β1), alpha 2 beta 1 (α2β1), alpha 10 beta 1 (α10β1) and alpha 11 beta 1 (α11β1), though there are 24 different types of integrins (formed from 18 α and 8 β subunits) in humans. These four collagen receptor integrins, (α1β1, α2β1, α10β1 and α11β1) are classified under the integrins α1 domain subgroup [17,18,19]. Though the four integrins are reported as collagen-binding receptors, they are expressed in a different location and carry unique signals, for instance, the primary site of integrin expression as follows: fibroblasts and mesenchymal tissues for α1β1 integrin; platelets, epithelium, fibroblasts, and mesenchymal tissues for α2β1 integrin; cartilage and chondrocytes for α10β1 integrin; and periodontal ligaments for α11β1. These integrins transfer the signals in a bidirectional way from outside to inside and vice versa [13]. The reorganization of collagenous matrix by integrins α1β1, α2β1, and α11β1 during collagen contraction is an important process in wound healing [20,21,22]. Among the four types, the signaling mechanism of α1β1 and α2β1 integrins has especially been reported. These integrins bind to both collagen types I and IV; however, their affinities differ: α1β1 has a higher affinity for collagen type IV, while α2β1 preferentially binds to collagen type I [13,23].
Integrins are well-known cell surface heterodimeric receptors distributed to almost all biological cells, which regulate the development and pathological processes of organs. Integrins constitute a major group of receptors for extracellular matrix components, including collagens. Integrins are widely distributed in organs such as skin, kidney, lungs, muscles, inner ears, eyes, heart, blood vessels, vascular endothelium, heart, gut (Peyer’s patches), lymphatic endothelium, mesenchymal tissue, cartilage and periodontal ligaments; and cells such as macrophage, platelets, fibroblasts, immune cells, bone cells, cancer cells, leucocytes (all types), eosinophils, chondrocytes, embryonic stem cells and so on [1]. Integrins play an essential role in regulating cell signals, migration, survival and differentiation [2][3]. At present, four types of collagen-binding integrins have been identified, namely alpha 1 beta 1 (α1β1), alpha 2 beta 1 (α2β1), alpha 10 beta 1 (α10β1) and alpha 11 beta 1 (α11β1), though there are 24 different types of integrins (formed from 18 α and 8 β subunits) in humans. These four collagen receptor integrins, (α1β1, α2β1, α10β1 and α11β1) are classified under the integrins α1 domain subgroup [4][5][6]. Though the four integrins are reported as collagen-binding receptors, they are expressed in a different location and carry unique signals, for instance, the primary site of integrin expression as follows: fibroblasts and mesenchymal tissues for α1β1 integrin; platelets, epithelium, fibroblasts, and mesenchymal tissues for α2β1 integrin; cartilage and chondrocytes for α10β1 integrin; and periodontal ligaments for α11β1. These integrins transfer the signals in a bidirectional way from outside to inside and vice versa [2]. The reorganization of collagenous matrix by integrins α1β1, α2β1, and α11β1 during collagen contraction is an important process in wound healing [7][8][9]. Among the four types, the signaling mechanism of α1β1 and α2β1 integrins has especially been reported. These integrins bind to both collagen types I and IV; however, their affinities differ: α1β1 has a higher affinity for collagen type IV, while α2β1 preferentially binds to collagen type I [2][10].
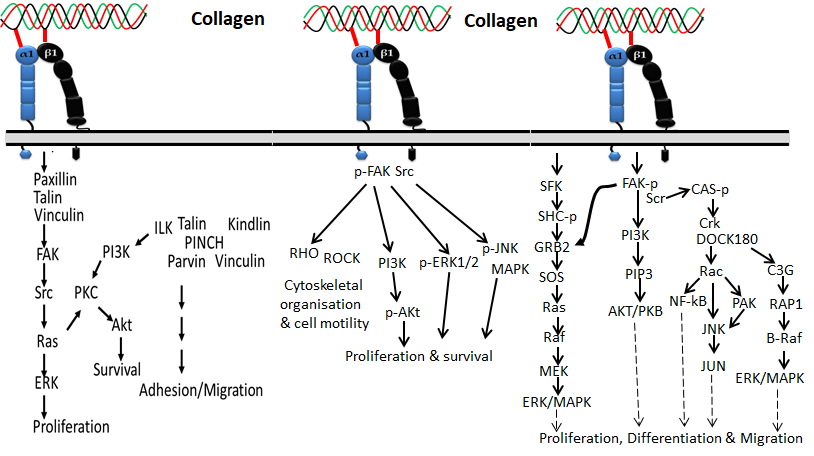
Figure 1 Collagen integrin signals in the normal physiological function of cells
Based on the available evidence from the literature, integrin α1β1 was first discovered by Hemler et al. [24][11] and is mainly located on mesenchymal, immune and epithelial cells, which preferentially bind collagen I, collagen IV, collagen VI, collagen IX, collagen XIII and collagen XVI and also other types of fibrillary collagens [25,26,27][12][13][14] via the MIDAS motif in the α subunit I domain. Integrin α1β1 is commonly expressed in activated lymphocytes, liver, dermis, visceral, kidney, heart, ganglia, microvascular endothelium, and some vascular smooth muscles [28,29,30,31,32][15][16][17][18][19]. Collagen binding with the integrin α1β1 receptor regulates the proliferation of living cells, MMP expression and collagen synthesis.
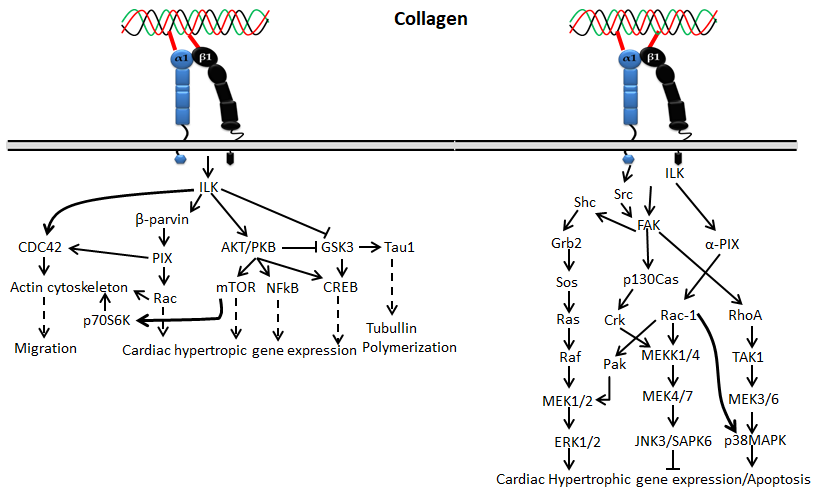
Figure 2. Collagen integrin signals in cardiac hypertrophic gene expression
The α2β1 integrin, also known as VLA-2, GPIa-IIa, and CD49b, was first identified as an extracellular matrix receptor for collagens and/or laminins [33,34][20][21]. Integrin α2β1 has been reported to be one of the main collagen-binding integrins present in bone, skin and other internal organs that comprise epithelial cells, immune cells, platelets, fibroblasts, chondrocytes and mesenchymal cells. [23,35,36,37][10][22][23][24]. Among collagens, fibrillar isoforms of collagen I–III, V and XI could preferentially interact with the α2β1 integrin. Not only fibrillar isoforms of collagen, but also the beaded-filament-forming collagen VI, the transmembrane collagen XIII [38][25], collagen XVI [26][13] and network-forming collagen IV [39][26] are also recognized by integrin α2β1. The interaction of collagen with integrin α2β1 is synchronized by the collagen sequence GFOGER [40,41,42][27][28][29]. It was reported that the interaction of collagens such as type I, II and XI with platelet integrin α2β1 is materialized by the GFOGER motif even without stimulators [43][30].
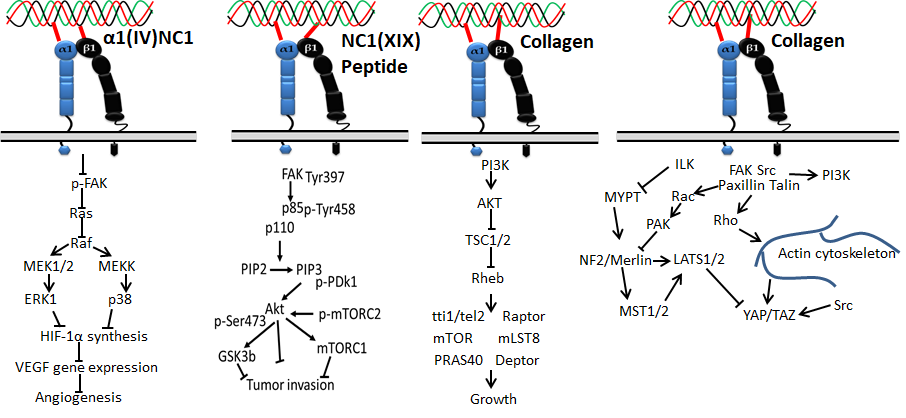
Figure 3. Collagen integrin signals in cancer
Integrin α10β1 is a primary receptor of collagen type II that was first identified on chondrocytes in 1998 [44][31]. Since cartilage is a major site of collagen type II, integrin α10β1 is most abundant in cartilage tissues of the vertebrae, joints, ribs, bronchi and trachea, and thus the expression pattern is unique compared to other integrins. Later, it was identified that integrin α10β1 also binds with other types of collagen-like collagens I, IV and VI. It is expressed on cardiac cells, chondrocytes, perichondrium, endosteum, bone lining cells, fascia lining skeletal muscle fibers and periosteum.
Integrin α11β1 was identified on human fetal myotubes in 1995 by Gullberg et al. [45][32]. Like integrin α2β1, integrin α11β1 also binds with fibrillar collagen such as collagen I and XIII. It is expressed in organs such as embryo, muscles, and bone, and cells such as mesenchymal stem cells, myocytes, fibroblasts, bone cells, and monocytes [46][33]. Aside from the four integrins mentioned above, integrin α6β4 and integrin α5β3 were also recently reported to interact with collagen XVII and Noncollagenous domain (NC1) of type XIX collagen, respectively [47,48][34][35].
2. Receptor Tyrosine Kinases (DDR)
2. Receptor Tyrosine Kinases (DDR)
Receptor tyrosine kinase is also known as the discoidin domain receptor (DDR1 and DDR2), which plays an important role in the development and growth of organs and is generally activated by binding with different types of collagens such as collagen I–V [49,50][36][37]. Since it regulates organs’ growth, any impairment of DDRs may cause disorders in several organs [51][38]. The activation of the receptor is generally slow and prolonged by collagen stimulation through binding with tyrosine residue autophosphorylation, followed by receptor internalization [52,53][39][40]. Empirical evidence claims that binding of collagen with discoidin-homology domain (DD) induces autophosphorylation of the receptor through upregulated N-cadherin expression and Src signaling [49,51,54,55][36][38][41][42].
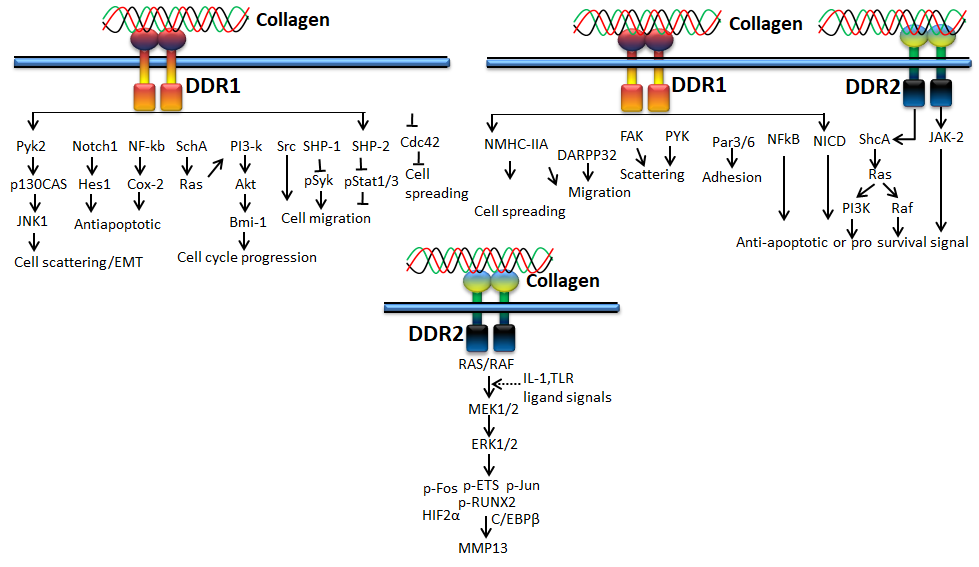
Figure 4. Collagen
DDR signals in cell proliferation and survival
DDR1, a transmembrane tyrosine kinase receptor, is an important collagen receptor for intracellular signals for cell proliferation, survival, homing, and colonization and is expressed in many cells and organs [56][43]. Triggering phosphorylation of tyrosine kinase domains through dimerization in DDR1 can activate various signaling pathways such as MAPK/ER kinase, P38 kinase JNK or PI3 kinase pathways. Notably, in normal conditions, collagen does not interact with DDR1, though collagen is most abundant in the extracellular matrix. However, DDR1 specifically interacts with collagen in cell proliferation, differentiation, migration, and inflammatory response during chronic diseases such as pulmonary, kidney and vascular infection, and is more specifically overexpressed in tumor state. The physiological function of DDR1 has been regulated by ADAM10-mediated ectodomain shedding [57][44]. Any imbalance of DDR1 leads to atherosclerosis, fibrosis, temporomandibular joint disorder osteoarthritis, and tumor [58,59,60][45][46][47]. On the other hand, DDR2 is mainly expressed in chondrocytes and is involved in the development of bone and cartilages through increasing matrix metalloproteinase expression [61][48]. In addition to cartilage, DDR2 is involved in the pathological process of arthritis, wound healing, dwarfism and tumor [62,63,64][49][50][51]. More precisely, DDR1 binds to collagen type I and IV, whereas DDR2 interacts with collagen type I, II, and X.
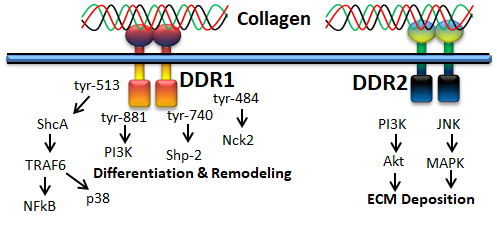
Figure 5. Collagen DDR signals in ECM deposition
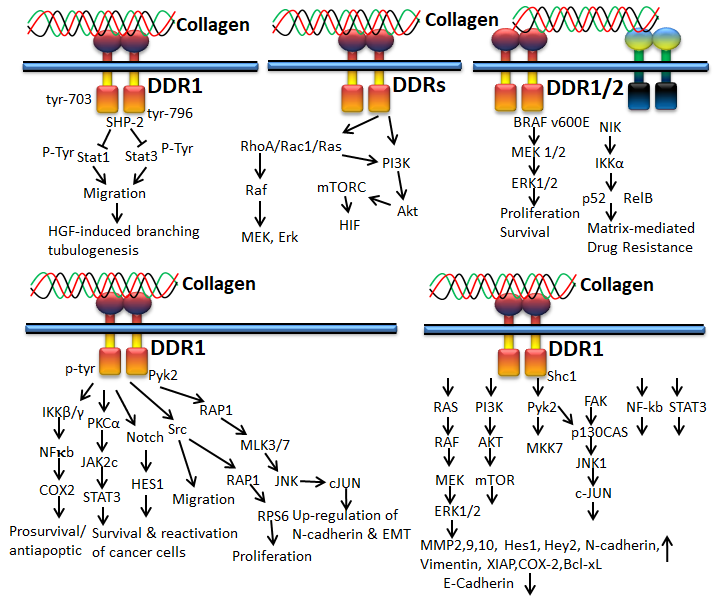
Figure 6. Collagen DDR signals in cancer
3. Immunoglobulin Receptor
Glycoprotein VI (GPVI) is an immunoglobulin-based transmembrane stimulatory receptor that is expressed in megakaryocytes and platelets and specifically binds with Gly-Pro-Hyp amino acid residues of collagen. The non-covalent interaction of GPVI with Fc receptor is attained by the presence of positively charged arginine in the transmembrane region. Additionally, the proline-rich motif of GPVI cytosolic tail selectively binds with the Src homology 3 (SH3) domain of the Src family tyrosine kinases Lyn and Fyn [65,66][52][53]. Inside-out signaling of GPVI releases stored mediators to activate integrins during thrombus growth, and GPVI signals can be controlled by immunoreceptor tyrosine-based inhibition motif (ITIM)-coupled receptors such as PECAM-1 (CD31) [67][54]. Studies also claim that binding of Syk to the FcR-γ chain initiates activation of Syk proteins, tyrosine phosphorylation and phospholipase C γ2 (PLCγ2) [67][54]. GPVI in platelets binds mainly with collagen during the process of blood coagulation [68][55].
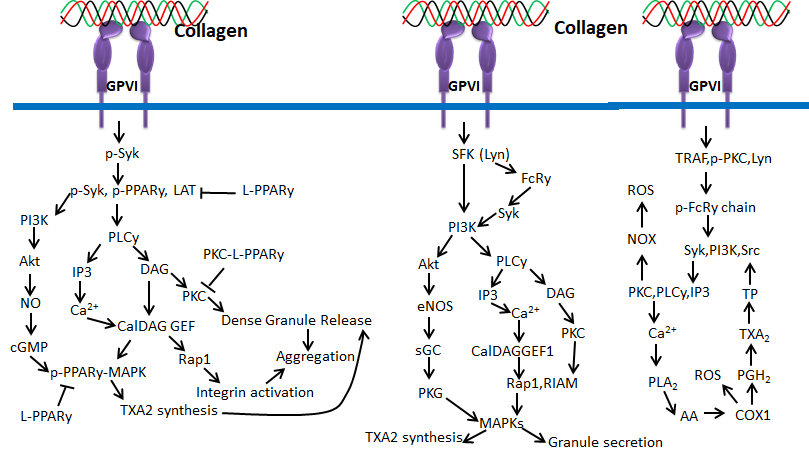
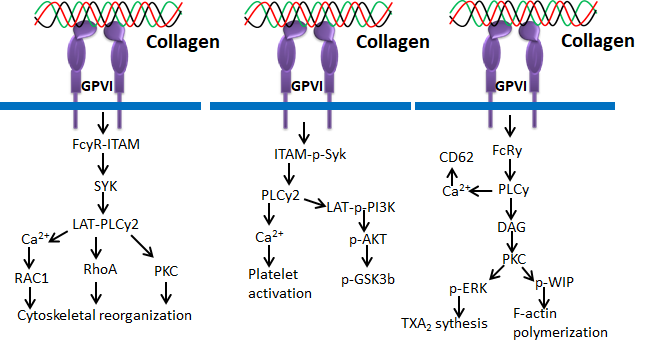
Figure 7. Collagen GPVI signals in platelets activation and ROS production
The G6B receptor belongs to the immunoglobulin (Ig) superfamily and is located in the MHC class III region. There are two types of receptor isoforms—G6B-A and G6B-B—with similar N-terminal and varying C-terminal cytoplasmic tails. This receptor binds with SHP-1 and SHP-2 through phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its cytoplasmic tail. This receptor accelerates SHP-1 and SHP-2 through ITIMs in its cytoplasmic domain in order to inhibit signaling pathways. G6B is articulated on platelets and megakaryocytes, and functions as a negative regulator of platelet function [69][56].
Human osteoclast-associated receptor (OSCAR) is another collagen receptor that belongs to the immunoglobulin (Ig) superfamily. This receptor is expressed in a wide range of myeloid cells and is specifically involved in osteoclast growth induction for bone resorption. The level of OSCAR expression is higher during osteoclastogenesis towards bone resorption, which could be achieved through triggering leukocyte receptor complex and FcRγ. Therefore, OSCAR is mainly essential for the differentiation of osteoclast, since it acts as a vital co-stimulatory receptor for osteoclast function and formation [70,71,72,73][57][58][59][60]. Collagen binding to OSCAR leads to conscription of immunoreceptor tyrosine-based activation motifs (ITAM) containing FcRγ chains. This process further activates the downstream effect of calcium signaling, which is essentially important for the activation of an osteoclastogenic transcription factor such as the nuclear factor of activated T-cells (NFAT) c1.
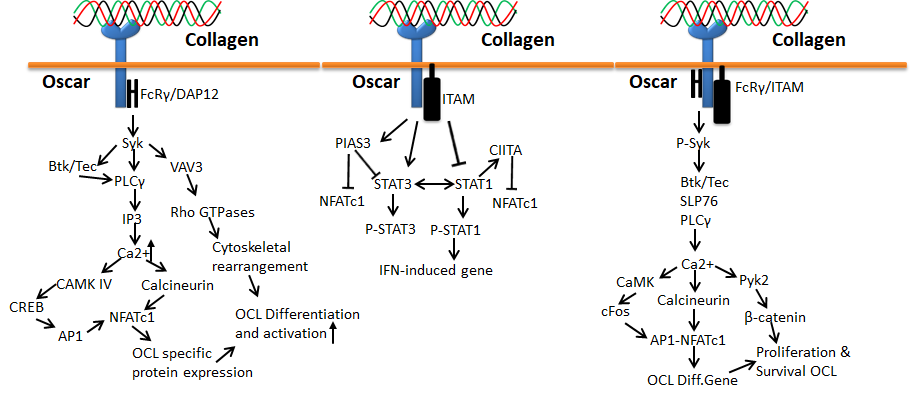
Figure 8. Collagen Oscar signals in bone
4. Leukocyte Receptor Complex (LRC)
LRC is a typical group of receptors primarily expressed in immune cells and plays a diverse role in immune functions such as autoimmunity, antiviral immunity, and graft tolerance [61][62]. The stimulatory receptors contain short cytoplasmic tails and produce positive cues through ITAM of adapter proteins such as DAP10, DAP12 and FcRγ, whereas the inhibitory receptors act through immunoreceptor tyrosine-based inhibitory motifs (ITIM) with long cytoplasmic tails. Interestingly, both stimulatory and inhibitory receptors of LRC could efficiently bind with collagen. The stimulatory and inhibitory receptors of this family include LAIR-1, OSCAR and GPVI [29][53][57][63]. Among these three receptors, the OSCAR and GPVI were already discussed in the previous section. LAIR-1 is an inhibitory receptor actuated by triple-helical collagen as a ligand through interaction with the collagen-related peptide, triplet (glycine-proline-hydroxyproline GPO)10 [64]. It was first identified in platelets and megakaryocytes [63][64][65]. LAIR-1 is also expressed in osteoclast precursors to downregulate osteoclastogenesis [66]. The phosphorylation of LAIR-1 containing two ITIMs recruits phosphatases SHP-1 and 2 and directly dephosphorylates Zap70, PLCγ and Syk, inhibiting ITAM-mediated stimulation of protein kinases and calcium signaling [63][64].
5. Other Receptors
5.1. Fibronectin
Fibronectin (FBN) is an abundant high MW glycoprotein in the extracellular matrix. FBN has multiple binding domains for several biomolecules such as collagens, proteoglycans and TGF-β; the first isolated domain of FBN, consisting of the 6FNI1–2FNII7–9FNI repeats near the N-terminus, is specific for collagen [67][68]. The FBN has a higher affinity to gelatin (denatured collagen) than collagen, since it plays an essential role in tissue growth and wound repair [69][70]. The binding site of FBN in collagen combined with the cleavage site of matrix metalloproteinase (MMP)-1 and the Arg-Gly-Asp (RGD) motif recognition sited in the 10FNIII of the domain is fundamental for cell adhesion [71][72]. The main function of FBN is to promote cell–basement membrane attachment, macrophage function, fibroblast migration, nerve regeneration, clot stabilization, cell-to-cell adhesion, pathogen (virus, fungus, bacteria, and protozoa) binding to mammalian cells and extracellular matrix and embryogenesis.
5.2. Vitronectin
Another glycoprotein of the extracellular matrix that binds with collagen is Vitronectin (VTN), which belongs to the hemopexin family and was first identified in serum, named as serum spreading factor [73]. It is mainly distributed in the extracellular matrix, blood serum, platelets and bone. The main function of VTN is to promote cell proliferation, adhesion, immune defense, hemostasis and fibrinolysis [74][75]. Unlike FBN, VTN has a greater affinity to native fibrillar triple-helical collagens than denatured collagen (gelatin) [76]. Empirical evidence proves the inhibitory effect of VTN towards FBN interaction with collagen, proposing that both proteins (VTN and FBN) have competitive attraction at similar binding sites on collagen. Interestingly, the interaction between VTN and collagen is highly regulated by the glycosylation status of VTN, which controls cell migration and adhesion in the tissues [77].
4. Leukocyte Receptor Complex (LRC)
LRC is a typical group of receptors primarily expressed in immune cells and plays a diverse role in immune functions such as autoimmunity, antiviral immunity, and graft tolerance [74,75]. The stimulatory receptors contain short cytoplasmic tails and produce positive cues through ITAM of adapter proteins such as DAP10, DAP12 and FcRγ, whereas the inhibitory receptors act through immunoreceptor tyrosine-based inhibitory motifs (ITIM) with long cytoplasmic tails. Interestingly, both stimulatory and inhibitory receptors of LRC could efficiently bind with collagen. The stimulatory and inhibitory receptors of this family include LAIR-1, OSCAR and GPVI [42,66,70,76]. Among these three receptors, the OSCAR and GPVI were already discussed in the previous section. LAIR-1 is an inhibitory receptor actuated by triple-helical collagen as a ligand through interaction with the collagen-related peptide, triplet (glycine-proline-hydroxyproline GPO)10 [77]. It was first identified in platelets and megakaryocytes [76,77,78]. LAIR-1 is also expressed in osteoclast precursors to downregulate osteoclastogenesis [79]. The phosphorylation of LAIR-1 containing two ITIMs recruits phosphatases SHP-1 and 2 and directly dephosphorylates Zap70, PLCγ and Syk, inhibiting ITAM-mediated stimulation of protein kinases and calcium signaling [76,77].
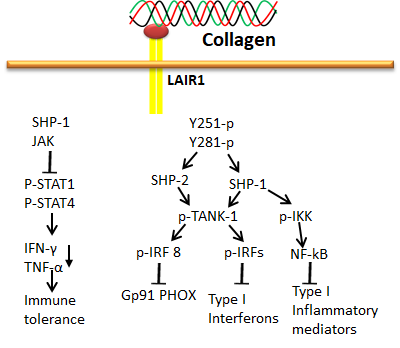
Figure 9. Collagen LAIR1 signals in immune tolerance
5.3. uPARAP
5. Other Receptors
5.1. Fibronectin
Fibronectin (FBN) is an abundant high MW glycoprotein in the extracellular matrix. FBN has multiple binding domains for several biomolecules such as collagens, proteoglycans and TGF-β; the first isolated domain of FBN, consisting of the 6FNI1–2FNII7–9FNI repeats near the N-terminus, is specific for collagen [80,81]. The FBN has a higher affinity to gelatin (denatured collagen) than collagen, since it plays an essential role in tissue growth and wound repair [82,83]. The binding site of FBN in collagen combined with the cleavage site of matrix metalloproteinase (MMP)-1 and the Arg-Gly-Asp (RGD) motif recognition sited in the 10FNIII of the domain is fundamental for cell adhesion [84,85]. The main function of FBN is to promote cell–basement membrane attachment, macrophage function, fibroblast migration, nerve regeneration, clot stabilization, cell-to-cell adhesion, pathogen (virus, fungus, bacteria, and protozoa) binding to mammalian cells and extracellular matrix and embryogenesis.
5.2. Vitronectin
Another glycoprotein of the extracellular matrix that binds with collagen is Vitronectin (VTN), which belongs to the hemopexin family and was first identified in serum, named as serum spreading factor [86]. It is mainly distributed in the extracellular matrix, blood serum, platelets and bone. The main function of VTN is to promote cell proliferation, adhesion, immune defense, hemostasis and fibrinolysis [87,88]. Unlike FBN, VTN has a greater affinity to native fibrillar triple-helical collagens than denatured collagen (gelatin) [89]. Empirical evidence proves the inhibitory effect of VTN towards FBN interaction with collagen, proposing that both proteins (VTN and FBN) have competitive attraction at similar binding sites on collagen. Interestingly, the interaction between VTN and collagen is highly regulated by the glycosylation status of VTN, which controls cell migration and adhesion in the tissues [90].
5.3. uPARAP
The urokinase plasminogen activator receptor-associated protein (uPARAP, also known as Endo180) is a multi-domain type I transmembrane glycoprotein that belongs to the mannose receptor family. It has several characteristic protein domains such as a series of 8–10 C-type lectin-like domains, cysteine-rich/ricin B-like domain, N-terminal and a fibronectin type-II domain. uPARAP/Endo180 located on the mesenchymal cell surface plays a major role in collagen internalization and turnover [85,91,92,93]. uPARAP/Endo180 is specifically involved in the primary adhesion of collagen to fibroblasts and speeds up the migration of fibroblasts on a fibrillar collagen matrix [91,92,94]. uPARAP/Endo180 is also highly expressed in bone cells such as osteocytes and osteoblasts at sites of intramembranous and endochondral ossification during development [95].
The urokinase plasminogen activator receptor-associated protein (uPARAP, also known as Endo180) is a multi-domain type I transmembrane glycoprotein that belongs to the mannose receptor family. It has several characteristic protein domains such as a series of 8–10 C-type lectin-like domains, cysteine-rich/ricin B-like domain, N-terminal and a fibronectin type-II domain. uPARAP/Endo180 located on the mesenchymal cell surface plays a major role in collagen internalization and turnover [72][78][79][80]. uPARAP/Endo180 is specifically involved in the primary adhesion of collagen to fibroblasts and speeds up the migration of fibroblasts on a fibrillar collagen matrix [78][79][81]. uPARAP/Endo180 is also highly expressed in bone cells such as osteocytes and osteoblasts at sites of intramembranous and endochondral ossification during development [82].
References
- Leitinger, B.; Hohenester, E. Mammalian collagen receptors. Matrix Biol. 2007, 26, 146–155.
- Hu, K.; Hu, M.; Xiao, Y.; Cui, Y.; Yan, J.; Yang, G.; Zhang, F.; Lin, G.; Yi, H.; Han, L.; et al. Preparation recombination human-like collagen/fibroin scaffold and promoting the cell compatibility with osteoblasts. J. Biomed. Mater. Res. Part A 2021, 109, 346–353.
- Lowell, C.A.; Mayadas, T.N. Overview: Studying integrins in vivo. In Methods in Molecular Biology; Sringer: Berlin/Heidelberg, Germany, 2011; pp. 369–397.
- Iwamoto, D.V.; Calderwood, D.A. Regulation of integrin-mediated adhesions. Curr. Opin. Cell Biol. 2015, 36, 41–47.
- Briesewitz, R.; Epstein, M.; Marcantonio, E. Expression of native and truncated forms of the human integrin alpha 1 subunit. J. Biol. Chem. 1993, 268, 2989–2996.
- Velling, T.; Kusche-Gullberg, M.; Sejersen, T.; Gullberg, D. cDNA cloning and chromosomal localization of human α11 integrin: A collagen-binding, i domain-containing, β1-associated integrin α-chain present in muscle tissues. J. Biol. Chem. 1999, 274, 25735–25742.
- Takada, Y.; Wayner, E.A.; Carter, W.G.; Hemler, M.E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J. Cell. Biochem. 1988, 37, 385–393.
- Klein, C.E.; Dressel, D.; Steinmayer, T.; Mauch, C.; Eckes, B.; Krieg, T.; Bankert, R.B.; Weber, L. Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J. Cell Biol. 1991, 115, 1427–1436.
- Gotwals, P.J.; Chi-Rosso, G.; Lindner, V.; Yang, J.; Ling, L.; Fawell, S.E.; Koteliansky, V.E. The alpha1beta1 integrin is expressed during neointima formation in rat arteries and mediates collagen matrix reorganization. J. Clin. Investig. 1996, 97, 2469–2477.
- Tiger, C.-F.; Fougerousse, F.; Grundström, G.; Velling, T.; Gullberg, D. α11β1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev. Biol. 2001, 237, 116–129.
- Xu, Y.; Gurusiddappa, S.; Rich, R.L.; Owens, R.T.; Keene, D.R.; Mayne, R.; Höök, A.; Höök, M. Multiple binding sites in collagen type I for the integrins α1β1 and α2β1. J. Biol. Chem. 2000, 275, 38981–38989.
- Hemler, M.; Glass, D.; Coblyn, J.S.; Jacobson, J.G. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J. Clin. Investig. 1986, 78, 696–702.
- Eble, J.; Golbik, R.; Mann, K.; Kühn, K. The alpha 1 beta 1 integrin recognition site of the basement membrane collagen molecule 2 alpha 2 (IV). EMBO J. 1993, 12, 4795–4802.
- Eble, J.A.; Kassner, A.; Niland, S.; Mörgelin, M.; Grifka, J.; Grässel, S. Collagen XVI harbors an integrin alpha1 beta1 recognition site in its C-terminal domains. J. Biol. Chem. 2006, 281, 25745–25756.
- Käpylä, J.; Jäälinoja, J.; Tulla, M.; Ylöstalo, J.; Nissinen, L.; Viitasalo, T.; Vehviläinen, P.; Marjomäki, V.; Nykvist, P.; Säämänen, A.-M.; et al. The fibril-associated collagen IX provides a novel mechanism for cell adhesion to cartilaginous matrix. J. Biol. Chem. 2004, 279, 51677–51687.
- Duband, J.-L.; Belkin, A.M.; Syfrig, J.; Thiery, J.P.; Koteliansky, V.E. Expression of alpha 1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development 1992, 116, 585–600.
- Gardner, H.; Kreidberg, J.; Koteliansky, V.; Jaenisch, R. Deletion of integrin α1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev. Biol. 1996, 175, 301–313.
- Hertle, M.D.; Adams, J.C.; Watt, F.M. Integrin expression during human epidermal development in vivo and in vitro. Development 1991, 112, 193–206.
- Korhonen, M.; Ylänne, J.; Laitinen, L.; Virtanen, I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J. Cell Biol. 1990, 111, 1245–1254.
- Terracio, L.; Rubin, K.; Gullberg, D.; Balog, E.; Carver, W.; Jyring, R.; Borg, T.K. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ. Res. 1991, 68, 734–744.
- Hemler, M.E. VLA proteins in the integrin family: Structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 1990, 8, 365–400.
- Hemler, M.E.; Jacobson, J.G.; Brenner, M.B.; Mann, D.; Strominger, J.L. VLA-1: A T cell surface antigen which defines a novel late stage of human T cell activation. Eur. J. Immunol. 1985, 15, 502–508.
- Helfrich, M.; Nesbitt, S.; Lakkakorpi, P.; Barnes, M.; Bodary, S.; Shankar, G.; Mason, W.; Mendrick, D.; Väänänen, H.; Horton, M. β1 integrins and osteoclast function: Involvement in collagen recognition and bone resorption. Bone 1996, 19, 317–328.
- Rodan, S.; Rodan, G. Integrin function in osteoclasts. J. Endocrinol. 1997, 154, S47–S56.
- Parks, W.C. What is the α2β1 integrin doing in the epidermis? J. Investig. Dermatol. 2007, 127, 264–266.
- Hägg, P.; Rehn, M.; Huhtala, P.; Väisänen, T.; Tamminen, M.; Pihlajaniemi, T. Type XIII collagen is identified as a plasma membrane protein. J. Biol. Chem. 1998, 273, 15590–15597.
- Kern, A.; Eble, J.; Golbik, R.; Kühn, K. Interaction of type IV collagen with the isolated integrins α1β1 and α2β1. Eur. J. Biochem. 1993, 215, 151–159.
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J. Biol. Chem. 1998, 273, 33287–33294.
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The Collagen-binding A-domains of Integrins α1β1 and α2β1recognize the same specific amino acid sequence, GFOGER, in native (Triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40.
- Raynal, N.; Hamaia, S.W.; Siljander, P.R.-M.; Maddox, B.; Peachey, A.R.; Fernandez, R.; Foley, L.J.; Slatter, D.A.; Jarvis, G.E.; Farndale, R.W. Use of synthetic peptides to locate novel integrin α2β1-binding motifs in human collagen III. J. Biol. Chem. 2006, 281, 3821–3831.
- Siljander, P.R.-M.; Hamaia, S.; Peachey, A.R.; Slatter, D.A.; Smethurst, P.A.; Ouwehand, W.H.; Knight, C.G.; Farndale, R.W. Integrin activation state determines selectivity for novel recognition sites in fibrillar collagens. J. Biol. Chem. 2004, 279, 47763–47772.
- Camper, L.; Hellman, U.; Lundgren-Åkerlund, E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998, 273, 20383–20389.
- Gullberg, D.; Velling, T.; Sjöberg, G.; Sejersen, T. Up-regulation of a novel integrin α-chain (αmt) on human fetal myotubes. Dev. Dyn. 1995, 204, 57–65.
- Garnotel, R.; Rittié, L.; Poitevin, S.; Monboisse, J.-C.; Nguyen, P.; Potron, G.; Maquart, F.-X.; Randoux, A.; Gillery, P. Human blood monocytes interact with type I collagen through αXβ2 integrin (CD11c-CD18, gp150-95). J. Immunol. 2000, 164, 5928–5934.
- Oudart, J.-B.; Doué, M.; Vautrin, A.; Brassart, B.; Sellier, C.; Dupont-Deshorgue, A.; Monboisse, J.-C.; Maquart, F.-X.; Brassart-Pasco, S.; Ramont, L. The anti-tumor NC1 domain of collagen XIX inhibits the FAK/PI3K/Akt/mTOR signaling pathway through αvβ3 integrin interaction. Oncotarget 2016, 7, 1516–1528.
- Löffek, S.; Hurskainen, T.; Jackow, J.; Sigloch, F.C.; Schilling, O.; Tasanen, K.; Bruckner-Tuderman, L.; Franzke, C.-W. Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PLoS ONE 2014, 9, e87263.
- Shrivastava, A.; Radziejewski, C.; Campbell, E.; Kovac, L.; McGlynn, M.; Ryan, T.E.; Davis, S.; Goldfarb, M.P.; Glass, D.J.; Lemke, G.; et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol. Cell 1997, 1, 25–34.
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23.
- Leitinger, B. Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 2014, 310, 39–87.
- Carafoli, F.; Hohenester, E. Collagen recognition and transmembrane signalling by discoidin domain receptors. Biochim. Biophys. Acta 2013, 1834, 2187–2194.
- Fu, H.L.; Valiathan, R.R.; Arkwright, R.; Sohail, A.; Mihai, C.; Kumarasiri, M.; Mahasenan, K.V.; Mobashery, S.; Huang, P.; Agarwal, G.; et al. Discoidin domain receptors: Unique receptor tyrosine kinases in collagen-mediated signaling. J. Biol. Chem. 2013, 288, 7430–7437.
- Shintani, Y.; Fukumoto, Y.; Chaika, N.; Svoboda, R.; Wheelock, M.J.; Johnson, K.R. Collagen I—Mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J. Cell Biol. 2008, 180, 1277–1289.
- Lu, K.K.; Trcka, D.; Bendeck, M.P. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc. Pathol. 2011, 20, 71–76.
- Valencia, K.; Ormazábal, C.; Zandueta, C.; Luis-Ravelo, D.; Antón, I.; Pajares, M.J.; Agorreta, J.; Montuenga, L.M.; Martínez-Canarias, S.; Leitinger, B.; et al. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces cell survival, homing, and colonization in lung cancer bone metastasis. Clin. Cancer Res. 2012, 18, 969–980.
- Shitomi, Y.; Thøgersen, I.B.; Ito, N.; Leitinger, B.; Enghild, J.J.; Itoh, Y. ADAM10 controls collagen signaling and cell migration on collagen by shedding the ectodomain of discoidin domain receptor 1 (DDR1). Mol. Biol. Cell 2015, 26, 659–673.
- Iwai, L.K.; Luczynski, M.T.; Huang, P.H. Discoidin domain receptors: A proteomic portrait. Cell. Mol. Life Sci. CMLS 2014, 71, 3269–3279.
- Schminke, B.; Muhammad, H.; Bode, C.; Sadowski, B.; Gerter, R.; Gersdorff, N.; Bürgers, R.; Monsonego-Ornan, E.; Rosen, V.; Miosge, N. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell. Mol. Life Sci. CMLS 2014, 71, 1081–1096.
- Ahmad, P.J.; Trcka, D.; Xue, S.; Franco, C.; Speer, M.Y.; Giachelli, C.M.; Bendeck, M.P. Discoidin domain receptor-1 deficiency attenuates atherosclerotic calcification and smooth muscle cell-mediated mineralization. Am. J. Pathol. 2009, 175, 2686–2696.
- Xu, L.; Peng, H.; Glasson, S.; Lee, P.L.; Hu, K.; Ijiri, K.; Olsen, B.R.; Goldring, M.B.; Li, Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007, 56, 2663–2673.
- Vogel, W. Discoidin domain receptors: Structural relations and functional implications. FASEB J. 1999, 13, S77–S82.
- Olaso, E.; Lin, H.C.; Wang, L.H.; Friedman, S.L. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair 2011, 4, 5.
- Labrador, J.P.; Azcoitia, V.; Tuckermann, J.; Lin, C.; Olaso, E.; Mañes, S.; Brückner, K.; Goergen, J.L.; Lemke, G.; Yancopoulos, G.; et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001, 2, 446–452.
- Suzuki-Inoue, K.; Tulasne, D.; Shen, Y.; Bori-Sanz, T.; Inoue, O.; Jung, S.M.; Moroi, M.; Andrews, R.K.; Berndt, M.C.; Watson, S.P. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. J. Biol. Chem. 2002, 277, 21561–21566.
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461.
- Watson, S.P.; Herbert, J.M.; Pollitt, A.Y. GPVI and CLEC-2 in hemostasis and vascular integrity. J. Thromb. Haemost. JTH 2010, 8, 1456–1467.
- Farndale, R.W. Collagen-induced platelet activation. Blood Cells Mol. Dis. 2006, 36, 162–165.
- Newland, S.A.; Macaulay, I.C.; Floto, A.R.; de Vet, E.C.; Ouwehand, W.H.; Watkins, N.A.; Lyons, P.A.; Campbell, D.R. The novel inhibitory receptor G6B is expressed on the surface of platelets and attenuates platelet function in vitro. Blood 2007, 109, 4806–4809.
- Barrow, A.D.; Raynal, N.; Andersen, T.L.; Slatter, D.A.; Bihan, D.; Pugh, N.; Cella, M.; Kim, T.; Rho, J.; Negishi-Koga, T.; et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Investig. 2011, 121, 3505–3516.
- Haywood, J.; Qi, J.; Chen, C.C.; Lu, G.; Liu, Y.; Yan, J.; Shi, Y.; Gao, G.F. Structural basis of collagen recognition by human osteoclast-associated receptor and design of osteoclastogenesis inhibitors. Proc. Natl. Acad. Sci. USA 2016, 113, 1038–1043.
- Zhou, L.; Hinerman, J.M.; Blaszczyk, M.; Miller, J.L.; Conrady, D.G.; Barrow, A.D.; Chirgadze, D.Y.; Bihan, D.; Farndale, R.W.; Herr, A.B. Structural basis for collagen recognition by the immune receptor OSCAR. Blood 2016, 127, 529–537.
- Kim, N.; Takami, M.; Rho, J.; Josien, R.; Choi, Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 2002, 195, 201–209.
- Martin, A.M.; Kulski, J.K.; Witt, C.; Pontarotti, P.; Christiansen, F.T. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 2002, 23, 81–88.
- Barrow, A.D.; Trowsdale, J. The extended human leukocyte receptor complex: Diverse ways of modulating immune responses. Immunol. Rev. 2008, 224, 98–123.
- Meyaard, L. The inhibitory collagen receptor LAIR-1 (CD305). J. Leukoc. Biol. 2008, 83, 799–803.
- Lebbink, R.J.; de Ruiter, T.; Adelmeijer, J.; Brenkman, A.B.; van Helvoort, J.M.; Koch, M.; Farndale, R.W.; Lisman, T.; Sonnenberg, A.; Lenting, P.J.; et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006, 203, 1419–1425.
- Coxon, C.H.; Sadler, A.J.; Huo, J.; Campbell, R.D. An investigation of hierachical protein recruitment to the inhibitory platelet receptor, G6B-b. PLoS ONE 2012, 7, e49543.
- Zhang, Y.; Ding, Y.; Huang, Y.; Zhang, C.; Boquan, J.; Ran, Z. Expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) on osteoclasts and its potential role in rheumatoid arthritis. Clinics 2013, 68, 475–481.
- Ruoslahti, E.; Hayman, E.G.; Kuusela, P.; Shively, J.E.; Engvall, E. Isolation of a tryptic fragment containing the collagen-binding site of plasma fibronectin. J. Biol. Chem. 1979, 254, 6054–6059.
- Ingham, K.; Brew, S.; Migliorini, M. Further Localization of the Gelatin-binding Determinants within Fibronectin: Active fragments devoid of type ii homologous repeat modules. J. Biol. Chem. 1989, 264, 16977–16980.
- McDONALD, J.A.; Kelley, D.G.; Broekelmann, T.J. Role of fibronectin in collagen deposition: Fab’to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 1982, 92, 485–492.
- Engvall, E.; Ruoslahti, E.; Miller, E. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J. Exp. Med. 1978, 147, 1584–1595.
- Pierschbacher, M.D.; Ruoslahti, E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc. Natl. Acad. Sci. USA 1984, 81, 5985–5988.
- Johansson, S.; Svineng, G.; Wennerberg, K.; Armulik, A.; Lohikangas, L. Fibronectin-integrin interactions. Front. Biosci. 1997, 2, d126–d146.
- Barnes, D.; Wolfe, R.; Serrero, G.; McClure, D.; Sato, G. Effects of a serum spreading factor on growth and morphology of cells in serum-free medium. J. Supramol. Struct. 1980, 14, 47–63.
- Hayman, E.G.; Pierschbacher, M.D.; Ohgren, Y.; Ruoslahti, E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc. Natl. Acad. Sci. USA 1983, 80, 4003–4007.
- Seiffert, D.; Schleef, R.R. Two functionally distinct pools of vitronectin (Vn) in the blood circulation: Identification of a heparin-binding competent population of Vn within platelet alpha-granules. Blood 1996, 88, 552–560.
- Gebb, C.; Hayman, E.G.; Engvall, E.; Ruoslahti, E. Interaction of vitronectin with collagen. J. Biol. Chem. 1986, 261, 16698–16703.
- Zeltz, C.; Orgel, J.; Gullberg, D. Molecular composition and function of integrin-based collagen glues—Introducing COLINBRIs. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2533–2548.
- Engelholm, L.H.; List, K.; Netzel-Arnett, S.; Cukierman, E.; Mitola, D.J.; Aaronson, H.; Kjøller, L.; Larsen, J.K.; Yamada, K.M.; Strickland, D.K.; et al. uPARAP/Endo180 is essential for cellular uptake of collagen and promotes fibroblast collagen adhesion. J. Cell Biol. 2003, 160, 1009–1015.
- Jürgensen, H.J.; Madsen, D.H.; Ingvarsen, S.; Melander, M.C.; Gårdsvoll, H.; Patthy, L.; Engelholm, L.H.; Behrendt, N. A novel functional role of collagen glycosylation: Interaction with the endocytic collagen receptor uparap/ENDO180. J. Biol. Chem. 2011, 286, 32736–32748.
- Jürgensen, H.J.; Johansson, K.; Madsen, D.H.; Porse, A.; Melander, M.C.; Sørensen, K.R.; Nielsen, C.; Bugge, T.H.; Behrendt, N.; Engelholm, L.H. Complex determinants in specific members of the mannose receptor family govern collagen endocytosis. J. Biol. Chem. 2014, 289, 7935–7947.
- Madsen, D.H.; Engelholm, L.H.; Ingvarsen, S.; Hillig, T.; Wagenaar-Miller, R.A.; Kjøller, L.; Gårdsvoll, H.; Høyer-Hansen, G.; Holmbeck, K.; Bugge, T.H. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J. Biol. Chem. 2007, 282, 27037–27045.
More
