Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Paramita Basu.
Nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) is a transcription factor encoded by the NFE2L2 gene and is a member of the cap ‘n’ collar subfamily of basic region leucine zipper transcription factors.
- chemotherapy-induced peripheral neuropathy
- chronic constriction injury
- diabetic neuropathy
- Nrf2
- partial sciatic nerve ligation
1. Peripheral Neuropathic Pain and Erythroid 2 (NFE2)-Related Factor 2 (Nrf2)
Pharmacological and genetic studies report crosstalk between NF-κB and the transcription factor nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2), such that the absence of Nrf2 can exacerbate NF-κB activity leading to the increased release of cytokine production. In turn, NF-κB can modulate the transcription and activity of Nrf2 [19][1]. Nrf2 is the product of the NFE2L2 gene and a member of the cap ‘n’ collar subfamily of basic region leucine zipper (bZip) transcription factors. Nrf2 contains a bZip domain at the C-terminus that is responsible for the formation of heterodimers with other bZip proteins, such as small muscle aponeurosis fibromatosis (MAF) K, G, and F [21,22][2][3]. These heterodimers are the regulators of 250 human genes located at the regulatory enhancer sequence known as the antioxidant response element (ARE), which resembles the NFE2-binding motif [23][4].
During normal physiological conditions, Nrf2 is bound to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm. Keap1 was identified as a Nrf2-binding protein by employing the yeast two hybrid system in which the inhibitory Neh2 domain of Nrf2 was a bait [24][5]. Keap1 contains two protein binding domains, the BTB (bric-a-brac, tramtrack, broad-complex) domain in the N-terminal region and Kelch repeats in the C-terminal region, which is homologous to Drosophila actin-binding protein Kelch (Kelch repeat, double glycine repeat domain) that mediates the binding of Keap1 to the Neh2 domain of Nrf2 [25,26,27][6][7][8]. The BTB domain is responsible for the homodimerization and binding of Keap1 to Cullin (Cul) 3, which is a scaffold protein of Nrf2 ubiquitin ligase (E3). Nrf2 has a half-life of approximately 20 min before it is degraded by proteasomes, which is mediated by the polyubiquitination through the Keap1/Cul3 ubiquitin ligase. Therefore, the protein levels of Nrf2 remain low in many cell types under normal physiological conditions [28,29,30][9][10][11].
During stressful physiological conditions, Nrf2 is released from Keap1 and translocates into the nucleus where it heterodimerizes with the MAF proteins. The complex Nrf2–MAF binds to ARE, initiating the transcriptions of several cytoprotective genes, such as heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase1 (NQO1), superoxide dismutase (SOD), glutathione cysteine ligase, glutathione S-transferases, and catalase (Figure 1) [31,32,33][12][13][14]. Thus, Nrf2 provides protection against oxidative stress. In support, Nrf2 knockout mice are highly susceptible to oxidative stress-related chemical toxicity and disease [34[15][16][17][18][19][20][21][22],35,36,37,38,39,40,41], leading researchers to postulate that targeting Nrf2′s protective role against oxidative stress and mitochondrial dysfunction [42,43,44,45][23][24][25][26] may provide a novel target for alleviating neuropathic pain. Here, wresarchers discuss pre-clinical evidence across several animal models of neuropathic pain of the therapeutic potential of targeting Nrf2 signaling and Nrf2 inducers.
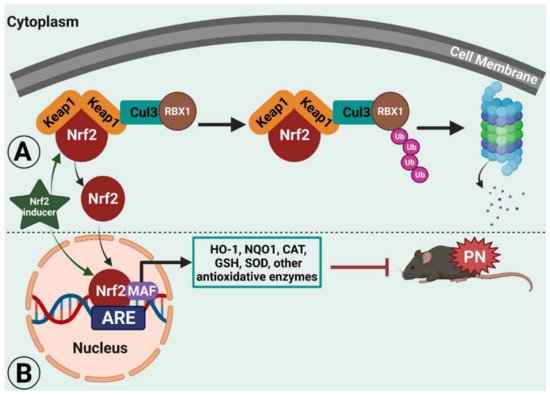
Figure 1. Illustrated working model of nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) signaling in rodent peripheral neuropathy (PN). Under normal physiological conditions, Nrf2 remains bound to Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm, which ultimately leads to proteasomal degradation.
2. Chemotherapy-Induced Peripheral Neuropathy (CIPN)
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most common neuropathies caused by antineoplastic agents [82][27], including platinum-based chemotherapeutic agents, taxanes, thalidomide and its analogues, and ixabepilone [83][28]. Oxaliplatin and paclitaxel are known to induce severe neuropathy during or immediately after the drug infusion [84][29]. The common symptoms of CIPN include “pins-and-needles” sensations, heat, burning, pain, as well as motor discoordination and muscle weakness [85][30]. Oxaliplatin is a third-generation platinum-derived chemotherapeutic agent that is used to treat colorectal and other cancers [86][31]. However, the beneficial effects of oxaliplatin and paclitaxel must be weighed against the risk of neurological side effects and peripheral neuropathic disorders, which affect 85–95% of the patients exposed to paclitaxel [87,88][32][33].
Nrf2−/− knockout mice display severe mechanical and cold hypersensitivities. Yang et al. reported that oxaliplatin-induced neuropathy in Nrf2−/− knockout mice resulted in greater production of ROS, decreased mitochondrial membrane potential with abnormal release of intracellular calcium, higher cytochrome C-related apoptosis, and overexpression of transient receptor potential (TRP) ion channels. All of these effects were attenuated by activating Nrf2 signaling with the Nrf2 inducer sulforaphane [89][34]. Similarly, Miao et al. reported that paclitaxel impaired Nrf-ARE and SOD in the dorsal root ganglia (DRG) in parallel with the production of oxidative stress markers (8-isoprostaglandin F2α (8-iso PGF2α) and 8-hydroxy-2′-deoxyguanosine (8-OHdG)) and proinflammatory cytokines (interleukin-1 beta (IL-1β), IL-6 and TNF-α), likely contributing to neuropathic pain [90][35]. Again, activation of the Nrf2/HO-1 signaling pathway alleviated paclitaxel-induced neuropathic pain, with a single dose of oltipraz attenuating pain while repeated administration abolished pain [91][36]. Remarkably, the antinociceptive effect of oltipraz was reversed by the Nrf2 inhibitor trigonelline, implicating a major role for Nrf2 signaling in chemotherapy-induced neuropathic pain.
Interactions with other inflammatory mediators have also been implicated. Crosstalk between the peroxisome proliferator-activated receptor gamma (PPARγ) and Nrf2/HO-1 signaling pathway has also been reported and may inform therapy development. Zhou et al. found the PPARγ selective agonist rosiglitazone reduced pain and upregulated the expression of Nrf2 and HO-1 in the spinal cord of paclitaxel-treated rats [92][37]. Moreover, the analgesic activity of rosiglitazone was abolished by the application of the Nrf2 inhibitor trigonelline. In addition, the Nrf2-ARE signaling pathway can also be targeted by microRNA (miRNA) treatment. Treatment with an inhibitor of miR-155, a miRNA that regulates inflammation, restored the oxaliplatin-induced impairment of the Nrf2-antioxidant response and Nrf2-regulated NQO1 protein expression in the dorsal horn of male rats [93][38]. Inhibition of miR-155 led to the attenuation of NOX4 protein expression, other oxidative stress products (8-iso PGF2α/8-OHdG), and TRPA1 in the dorsal horn of oxaliplatin-treated rats [93][38]. These data support the potential inhibitory effect of miR-155 in chemotherapy-induced PN via the Nrf2-ARE signaling pathway.
Alkaloid levo-corydalmine [94][39], antioxidant alphalipoic acid [95][40], mitoquinone [96][41], endogenous fatty acid amide palmitoylethanolamide (PEA) in association with oxazoline forming the 2-pentadecyl-2-oxazoline of palmitoylethanolamide (PEA-OXA) [97][42], curcumin [98][43], quercetin [99][44], resveratrol [100][45], formononetin [101][46], berberine [102][47], dimethyl fumarate and its metabolite monomethyl fumarate [103][48], and oleuropein [104][49] can reduce pain and inflammation associated with CIPN by activating the Nrf2 pathway. In addition to these Nrf2 inducers, Zhang and Xu shed light on the effects of bromodomain-containing protein 4 (BRD4) on the alleviation of vincristine-induced PN [105][50]. The study reported that BRD4 inhibition significantly reduced oxidative stress in sciatic nerve tissues by activating Nrf2. They postulate that the reducing expression of BRD4 by genetic therapy or drug intervention could inhibit macrophage infiltration and reduce inflammation and oxidative stress, providing a novel therapeutic target to be developed for the treatment of CIPN.
In addition to the aforementioned preclinical studies, Zhao et al. reported that electroacupuncture intervention restored the impairment of Nrf2-ARE/SOD, Nrf2-regulated NQO1, inhibited oxidative stress products (8-iso PGF2α/8-OHdG), and thereby attenuated the mechanical and thermal hypersensitivities in rats treated with paclitaxel [106][51]. Therefore, the study confirms the use of electropuncture as an alternative treatment strategy to treat CIPN [106][51].
Table 1. Evidence of the effects of Nrf2 inducers in rodent models of chemotherapy-induced peripheral neuropathy (CIPN).
| Nrf2 Inducer | Animals (Sex, Strain) | Dose (mg/kg), Route of Administration | Mechanism of Action | Reference |
|---|---|---|---|---|
| PEA-OXA | Male Wistar rats | 10 mg/kg, oral | NF-κB/Nrf-2 pathway | [97][42] |
| Oleuropein | Male Wistar rats | Oleuropein—20 mg/kg, oral Combination—Oleuropein—20 mg/kg, oral + suvorexant—an orexin receptor antagonist—20 mg/kg, oral |
Nrf2 pathway | [104][49] |
| Curcumin | Male Sprague Dawley rats | 100 and 200 mg/kg, oral | Nrf2/HO-1 pathway | [98][43] |
| Mitoquinone | Male ICR mice | 2.5, 5 and 10 mg/kg, intragastric | Nrf2 pathway | [96][41] |
| Formononetin | Male C57BL/6 mice | 10 mg/kg, i.p. 10 µM on mouse ND7/23 neuron cells, colon cancer cells (CT-26), human colorectal carcinoma cells (Caco-2, DLD-1, and HCT-116), human lung adenocarcinoma cells (PC9, A649, H1975, and HCC8827), human lung squamous cell carcinoma cells (H520), and human pancreatic cancer cells (BxPC3 and Panc1)—in vitro |
Keap1-Nrf2-GSTP1 pathway | [101][46] |
| Resveratrol | Male Sprague Dawley rats | 7 and 14 mg/kg, oral | Nrf2/HO-1 pathway | [100][45] |
| Quercetin | Male Sprague Dawley rats | 25 and 50 mg/kg, oral | Nrf2/HO-1 pathway | [99][44] |
| Oltipraz | Male Sprague Dawley rats | 10, 50, 100 mg/kg/day, i.p. | Nrf2/HO-1 pathway | [91][36] |
| Rosiglitazone | Male Sprague Dawley rats | 5, 25, and 50 mg/kg, i.p. | Nrf2/HO-1 pathway | [92][37] |
| Levo-corydalmine | Male ICR mice | 5, 10, and 20 mg/kg, intragastric | Nrf2/HO-1/CO pathway | [94][39] |
| Berberine | Male Wistar rats | 10 and 20 mg/kg, i.p. | Nrf2 pathway | [102][47] |
| Alphalipoic acid | Male Sprague Dawley rats | 15, 30, and 60 mg/kg, i.p. | Nrf2 pathway | [95][40] |
| L-carnosine | Male and female Egyptian patients | 500 mg, oral in patients—clinical trial | Nrf2 pathway | [107][52] |
| Dimethyl fumarate and its metabolite monomethyl fumarate | Rat | 0.3, 1, 3, or 10 mM dimethyl fumarate or monomethyl fumarate on PC12 cell—a rat pheochromocytoma cell | Nrf2 pathway | [103][48] |
| Sulforaphane | Nrf2+/+ and Nrf2−/− C57BL/6 mice | 5 mg/kg, i.p. 10 µM on DRG neurons |
Nrf2 pathway | [89][34] |
Note. Table is organized based on the most recent to oldest publications.
References
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626.
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218.
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260.
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86.
- Ogura, T.; Tong, K.I.; Mio, K.; Maruyama, Y.; Kurokawa, H.; Sato, C.; Yamamoto, M. Keap1 is a forked-stem dimer structure with two large spheres enclosing the intervening, double glycine repeat, and C-terminal domains. Proc. Natl. Acad. Sci. USA 2010, 107, 2842–2847.
- Li, X.; Zhang, D.; Hannink, M.; Beamer, L.J. Crystal structure of the Kelch domain of human Keap1. J. Biol. Chem. 2004, 279, 54750–54758.
- Padmanabhan, B.; Tong, K.I.; Ohta, T.; Nakamura, Y.; Scharlock, M.; Ohtsuji, M.; Kang, M.-I.; Kobayashi, A.; Yokoyama, S.; Yamamoto, M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell 2006, 21, 689–700.
- He, X.; Chen, M.G.; Lin, G.X.; Ma, Q. Arsenic induces NAD (P) H-quinone oxidoreductase I by disrupting the Nrf2· Keap1· Cul3 complex and recruiting Nrf2· Maf to the antioxidant response element enhancer. J. Biol. Chem. 2006, 281, 23620–23631.
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139.
- Zhang, D.D.; Lo, S.-C.; Cross, J.V.; Templeton, D.J.; Hannink, M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004, 24, 10941–10953.
- Vasconcelos, A.R.; Dos Santos, N.B.; Scavone, C.; Munhoz, C.D. Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Front. Pharmacol. 2019, 10, 33.
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295.
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041.
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116.
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557.
- Ma, Q.; He, X. Molecular basis of electrophilic and oxidative defense: Promises and perils of Nrf2. Pharmacol. Rev. 2012, 64, 1055–1081.
- Chan, K.; Han, X.-D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616.
- Klaassen, C.D.; Reisman, S.A. Nrf2 the rescue: Effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 2010, 244, 57–65.
- Walters, D.M.; Cho, H.-Y.; Kleeberger, S.R. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: A potential role for Nrf2. Antioxid. Redox Signal. 2008, 10, 321–332.
- de la Vega, M.R.; Dodson, M.; Gross, C.; Mansour, H.M.; Lantz, R.C.; Chapman, E.; Wang, T.; Black, S.M.; Garcia, J.G.; Zhang, D.D. Role of Nrf2 and autophagy in acute lung injury. Curr. Pharmacol. Rep. 2016, 2, 91–101.
- Pajares, M.; Jiménez-Moreno, N.; Dias, I.H.; Debelec, B.; Vucetic, M.; Fladmark, K.E.; Basaga, H.; Ribaric, S.; Milisav, I.; Cuadrado, A. Redox control of protein degradation. Redox Biol. 2015, 6, 409–420.
- Wang, G.-Q.; Zhang, B.; He, X.-M.; Li, D.-D.; Shi, J.-S.; Zhang, F. Naringenin targets on astroglial Nrf2 to support dopaminergic neurons. Pharmacol. Res. 2019, 139, 452–459.
- Wati, S.M.; Matsumaru, D.; Motohashi, H. NRF2 pathway activation by KEAP1 inhibition attenuates the manifestation of aging phenotypes in salivary glands. Redox Biol. 2020, 36, 101603.
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916.
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017, 11, 543–553.
- Hershman, D.L.; Lacchetti, C.; Dworkin, R.H.; Lavoie Smith, E.M.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Gavin, P.; Lavino, A.; Lustberg, M.B. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 1941–1967.
- Banach, M.; Juranek, J.K.; Zygulska, A.L. Chemotherapy-induced neuropathies—A growing problem for patients and health care providers. Brain Behav. 2017, 7, e00558.
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 2019, 20, 1451.
- Brown, T.J.; Sedhom, R.; Gupta, A. Chemotherapy-induced peripheral neuropathy. JAMA Oncol. 2019, 5, 750.
- Carozzi, V.; Canta, A.; Chiorazzi, A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci. Lett. 2015, 596, 90–107.
- Gamelin, E.; Gamelin, L.; Bossi, L.; Quasthoff, S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: Current management and development of preventive measures. Proc. Semin. Oncol. 2002, 29, 21–33.
- Tofthagen, C.; Donovan, K.A.; Morgan, M.A.; Shibata, D.; Yeh, Y. Oxaliplatin-induced peripheral neuropathy’s effects on health-related quality of life of colorectal cancer survivors. Support. Care Cancer 2013, 21, 3307–3313.
- Yang, Y.; Luo, L.; Cai, X.; Fang, Y.; Wang, J.; Chen, G.; Yang, J.; Zhou, Q.; Sun, X.; Cheng, X. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic. Biol. Med. 2018, 120, 13–24.
- Miao, H.; Xu, J.; Xu, D.; Ma, X.; Zhao, X.; Liu, L. Nociceptive behavior induced by chemotherapeutic paclitaxel and beneficial role of antioxidative pathways. Physiol. Res. 2019, 68, 491–500.
- Zhou, Y.-Q.; Liu, D.-Q.; Chen, S.-P.; Chen, N.; Sun, J.; Wang, X.-M.; Cao, F.; Tian, Y.-K.; Ye, D.-W. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020, 41, 1041–1048.
- Zhou, Y.-Q.; Liu, D.-Q.; Chen, S.-P.; Chen, N.; Sun, J.; Wang, X.-M.; Li, D.-Y.; Tian, Y.-K.; Ye, D.-W. PPARγ activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 129, 110356.
- Miao, F.; Wang, R.; Cui, G.; Li, X.; Wang, T.; Li, X. Engagement of microRNA-155 in exaggerated oxidative stress signal and TRPA1 in the dorsal horn of the spinal cord and neuropathic pain during chemotherapeutic oxaliplatin. Neurotox. Res. 2019, 36, 712–723.
- Zhou, L.; Ao, L.; Yan, Y.; Li, C.; Li, W.; Ye, A.; Liu, J.; Hu, Y.; Fang, W.; Li, Y. Levo-corydalmine attenuates vincristine-induced neuropathic pain in mice by upregulating the Nrf2/HO-1/CO pathway to inhibit connexin 43 expression. Neurotherapeutics 2020, 17, 340–355.
- Sun, H.; Guo, X.; Wang, Z.; Wang, P.; Zhang, Z.; Dong, J.; Zhuang, R.; Zhou, Y.; Ma, G.; Cai, W. Alphalipoic acid prevents oxidative stress and peripheral neuropathy in nab-paclitaxel-treated rats through the Nrf2 signalling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 3142732.
- Chen, X.-J.; Wang, L.; Song, X.-Y. Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. 2020, 125, 110003.
- Campolo, M.; Lanza, M.; Paterniti, I.; Filippone, A.; Ardizzone, A.; Casili, G.; Scuderi, S.A.; Puglisi, C.; Mare, M.; Memeo, L. PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis. Int. J. Mol. Sci. 2021, 22, 3927.
- Yardım, A.; Kandemir, F.M.; Çomaklı, S.; Özdemir, S.; Caglayan, C.; Kucukler, S.; Çelik, H. Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem. Res. 2021, 46, 379–395.
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-κB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146.
- Recalde, M.; Miguel, C.A.; Noya-Riobó, M.V.; Gonzalez, S.L.; Villar, M.J.; Coronel, M.F. Resveratrol exerts anti-oxidant and anti-inflammatory actions and prevents oxaliplatin-induced mechanical and thermal allodynia. Brain Res. 2020, 1748, 147079.
- Fang, Y.; Ye, J.; Zhao, B.; Sun, J.; Gu, N.; Chen, X.; Ren, L.; Chen, J.; Cai, X.; Zhang, W. Formononetin ameliorates oxaliplatin-induced peripheral neuropathy via the KEAP1-NRF2-GSTP1 axis. Redox Biol. 2020, 36, 101677.
- Singh, J.; Saha, L.; Singh, N.; Kumari, P.; Bhatia, A.; Chakrabarti, A. Study of nuclear factor-2 erythroid related factor-2 activator, berberine, in paclitaxel induced peripheral neuropathy pain model in rats. J. Pharm. Pharmacol. 2019, 71, 797–805.
- Kawashiri, T.; Miyagi, A.; Shimizu, S.; Shigematsu, N.; Kobayashi, D.; Shimazoe, T. Dimethyl fumarate ameliorates chemotherapy agent-induced neurotoxicity in vitro. J. Pharmacol. Sci. 2018, 137, 202–211.
- Chen, H.; Ma, D.; Zhang, H.; Tang, Y.; Wang, J.; Li, R.; Wen, W.; Zhang, Y. Antinociceptive effects of oleuropein in experimental models of neuropathic pain in male rats. Korean J. Pain 2021, 34, 35.
- Zhang, K.; Xu, Y. Suppressing BRD4 exhibits protective effects against vincristine-induced peripheral neuropathy by alleviating inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2020, 532, 271–279.
- Zhao, X.; Liu, L.; Wang, Y.; Wang, G.; Zhao, Y.; Zhang, Y. Electroacupuncture enhances antioxidative signal pathway and attenuates neuropathic pain induced by chemotherapeutic paclitaxel. Physiol. Res. 2019, 68, 501–510.
- Yehia, R.; Saleh, S.; El Abhar, H.; Saad, A.S.; Schaalan, M. L-Carnosine protects against Oxaliplatin-induced peripheral neuropathy in colorectal cancer patients: A perspective on targeting Nrf-2 and NF-κB pathways. Toxicol. Appl. Pharmacol. 2019, 365, 41–50.
More
