Background: Polyphenols are a set of bioactive compounds commonly found in plants. These compounds are of great interest, as they have shown high antioxidant power and are correlated to many health benefits. Hence, traditional methods of extraction such as solvent extraction, Soxhlet extraction and novel extraction technologies such as ultrasound-assisted extraction and subcritical water extraction (SWE) have been investigated for the extraction of polyphenols. Scope and Approach: Generally, for traditional extractions, the total phenolic content (TPC) is highest at an extraction temperature of 60–80 °C. For this reason, polyphenols are regularly regarded as heat-labile compounds. However, in many studies that investigated the optimal temperature for subcritical water extraction (SWE), temperatures as high as 100–200 °C have been reported. These SWE extractions showed extremely high yields and antioxidant capacities at these temperatures. This paper aimed to examine the relevant literature to identify and understand the mechanisms behind this discrepancy. Results: Thermal degradation is the most common explanation for the degradation of polyphenols. This may be the case for specific or sub-groups of phenolic acids. The different extraction temperatures may have also impacted the types of polyphenols extracted. At high extraction temperatures, the formation of new compounds known as Maillard reaction products may also influence the extracted polyphenols. The selection of source material for extraction, i.e., the plant matrix, and the effect of extraction conditions, i.e., oxidation and light exposure, are also discussed. The overestimation of total phenolic content by the Folin–Ciocâlteu assay is also discussed. There is also a lack of consensus in TPC’s correlation to antioxidant activity.
Polyphenols are a set of bioactive compounds commonly found in plants. These compounds are of great interest, as they have shown high antioxidant power and are correlated to many health benefits. Hence, traditional methods of extraction such as solvent extraction, Soxhlet extraction and novel extraction technologies such as ultrasound-assisted extraction and subcritical water extraction (SWE) have been investigated for the extraction of polyphenols.
- polyphenols
- antioxidant
- bioactive compounds
- pressurised liquid extraction
1. Introduction
2. Thermal Degradation
| Source | Extraction | Temperatures Tested | Effect on Polyphenols | Reference |
|---|---|---|---|---|
| Red grape pomace peels | TSE | Dried at 60, 100 and 140 °C; freeze-dried samples served as controls | TPC ↓ at 100 °C | [35] |
| Grape seed flour (GSF) | TSE | Heated at 120, 150, 180, 210 or 240 °C | TPC ↓ above 180 °C TFC ↓ above 120 °C |
[34] |
| Black rice | TSE | Dried at 20, 40, 60, 80 and 100 °C | TFC ↓ above 40 °C TPC ↓ above 80 °C |
[30] |
| Spinach | PLE | Extractions between 50–190 °C | Flavonoids ↓ at 130 °C No decrease in TPC |
[41] |
| Black currants | TSE | Extractions between 20–60 °C | T ↑, TPC ↑ Anthocyanins ↓ above 45 °C. |
[16] |
| Hemp, flax, canola seed cakes | TSE | Extractions at 40, 50, 60, 70 °C | T ↑, TPC ↑ TFC ↓ above 60 °C in flax and canola seed cake TFC ↓ above 70 °C in hempseed cake |
[57] |
| Peach | TSE | Extractions between 25–70 °C | TFC ↓ above 60 °C TPC remains same between 25–70 °C |
[55] |
| Mango peels and seed | TSE | Extractions at 25, 50, and 75 °C | TFC ↓ at 50 and 75 °C | [58] |
| Red grape skin | PLE | Extractions between 20 to 140 °C | Anthocyanins ↓ above 100 °C TPC ↓ above 120 °C |
[62] |
| Elderberry, strawberry and black carrot | TSE | Heated at 95 °C | Anthocyanins ↓ | [64] |
| Red cabbage | TSE | Blanched at 94–96 °C | TPC ↑ at 94–96 °C Anthocyanins ↓ at 94–96 °C |
[63] |
3. Effects of Other Parameters on Traditional and PLE Extractions, i.e., Oxidation, Light Sensitivity, Heating Time and Enzymes
3.1. Extraction Conditions
3.2. Enzymes
3.3. Solvent Concentration and pH
4. Effects of Extraction Temperature on the Profiles of Polyphenols Extracted
Many studies have found that the extraction temperature significantly impacts the type of polyphenols extracted since various polyphenols degrade at different temperatures [36, 39,41,47,51,55,58,62,73–75].
Vergara-Salinas et al. [47] performed pressurised liquid extraction (PLE) on dried thyme and studied the effect of temperatures between 50 and 200 ◦C and the profile of polyphenols being extracted at each temperature using HPLC. The results showed that temperature had a significant effect on the polyphenol subclasses (Figure 1, Table 2). Hydroxyphenylpronanoic acid (HPPA) concentration increased by almost three times when the extraction temperature was 200 ◦C, while 100 ◦C was optimal for hydroxycinnamic acids, flavones, flavonols and total polyphenols. Additionally, higher extraction temperatures showed less diversity in the types of polyphenols extracted.

.
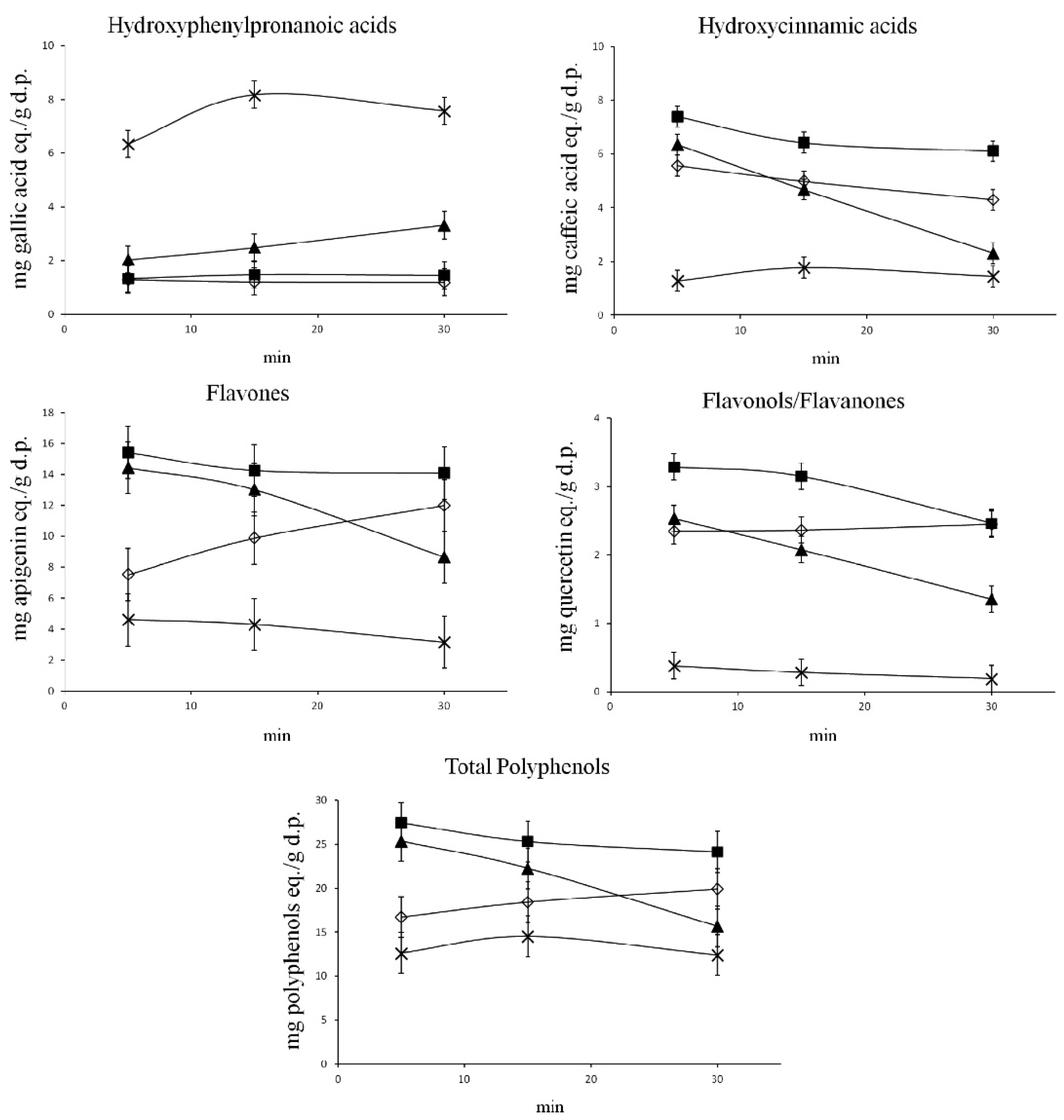
Figure 1. Impact of temperature on polyphenols and subclasses. Extraction temperatures: ( ) 50 ◦C; (■) 100 ◦C; (�) 150 ◦C; (×) 200 ◦C. Reprinted with permission from {Vergara-Salinas, J.R.; Pérez- Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929, doi:10.1021/jf3027759.}. Copyright {2012} American Chemical Society.
Table 2. Impact on individual phenolic compounds.
Source Extraction Effect Reference
Thyme PLE T ↑, hydroxyphenyl propanoic acids (HPPA) ↑, hydroxycinnamic acids ↓, flavones ↓, and flavanols ↓
[47]
Rosemary PLE T ↑, rosmanol ↓, carnosol ↓, carnosic acid ↑ [37]
|
TSE followed by acid hydrolysis
Ibañez et al. [37] performed a sequential extraction at 100, 150 and 200 ◦C and analysed the extracts using HPLC; they found that polar phenolic compounds were extracted at low temperatures while less polar phenolics were extracted at higher temperatures. The polarity of water was reduced at higher temperatures, allowing it to solvate the nonpolar com- pounds and extract them. As a result, depending on the extraction temperature, phenolic compounds can be extracted with high selectivity. Hossain et al. [45] studied the extraction of polyphenols from rosemary, oregano and marjoram and found that TPC increased as the temperature increased from 66 ◦C to 200 ◦C. However, at temperatures above 150 ◦C, rosmarinic acid and carnosic acid decreases (Table 2). As the AOA remained very high, the authors suggested that rosmarinic and carnosic acid broke down to compounds with higher antioxidant power.
Palma et al. [36] performed a PLE for polyphenols from grape peels and seeds. For grape seed extracts, compounds were detected at 150 ◦C that were not present at 50 ◦C and 100 ◦C. Mišan et al. [73] extracted polyphenols from parsley, buckthorn, mint, caraway and birch and also performed acid hydrolysis (HCL in 50% aqueous methanol heated to 90 ◦C for 2 h) and compared the results to unhydrolysed samples. With the assumption that hydrolysis of the phenols occurs at high temperatures, we can compare the results of this study to other papers performing extractions at high temperatures. The results show that depending on the plant, many polyphenols appear or disappear after hydrolysis. Table 2 below summarises studies that showed a shifting profile of phenolics depending on extraction conditions.
5
|
TSE followed by acid hydrolysis
| TSE followed by acid hydrolysis | |||
| All phenolic increased massively after hydrolysis, except caffeic acid | |||
| [ | 73 | ] | |
| Buckthorn | TSE followed by acid hydrolysis | Ferulic acid, myricetin, quercetin, naringenin, luteolin and apigenin appeared after hydrolysis. The content of the phenolics also increased with the exception of gallic acid, which slightly decreased. Vanillic acid was present in the normal extract but not present after hydrolysis | [73 |
|
TSE followed by acid hydrolysis
|
TSE followed by acid hydrolysis
|
TSE followed by acid hydrolysis
All phenolic increased massively after hydrolysis, except caffeic acid
Ferulic acid, myricetin, quercetin, naringenin, luteolin and apigenin appeared after hydrolysis. The content of the phenolics also increased with the exception of gallic acid, which slightly decreased. Vanillic acid was present in the normal extract but not present after hydrolysis
Myricetin, quercetin and kaempferol appeared after hydrolysis. Hydrolysis caused an increase in the content of gallic acid, protocatechuic acid and apigenin but a decrease in the content of caffeic acid and chlorogenic acid
Protocatechuic acid was found in the normal extract but not found after hydrolysis. Caffeic acid decreased
after hydrolysis
Quercetin appeared after hydrolysis. Increase in concentrations of all other phenolics after hydrolysis. Increase in gallic acid again explained by hydrolysis of galotannins
[73]
[73]
[73]
[73]
[73]
| ] | |||
| Birch | |||
| TSE followed by acid hydrolysis | Myricetin, quercetin and kaempferol appeared after hydrolysis. Hydrolysis caused an increase in the content of gallic acid, protocatechuic acid and apigenin but a decrease in the content of caffeic acid and chlorogenic acid | [73] | |
| Caraway | TSE followed by acid hydrolysis | Protocatechuic acid was found in the normal extract but not found after hydrolysis. Caffeic acid decreased after hydrolysis | [73] |
| Parsley | TSE followed by acid hydrolysis | Quercetin appeared after hydrolysis. Increase in concentrations of all other phenolics after hydrolysis. Increase in gallic acid again explained by hydrolysis of galotannins | [73] |
There is limited understanding of the composition, quantity and function of new com- pounds that are formed under PLE extractions at high temperatures. These compounds are commonly referred to as Maillard reaction products or MRPs. They have been reported to possess antioxidant activity and be toxic, mutagenic compounds [32,76,77]. Plaza et al. [78] found that newly formed compounds possessed AOA when extracted using PLE at 200 ◦C. Hossain et al. [45] showed that MRPs increased when the temperature increased from
150 ◦C to 200 ◦C, as did the TPC and AOA. With extraction temperatures above 150 ◦C, studies have reported the formation of MRPs and an increase in AOA [45,50,78,79]. Due to the possible toxic effects from MRPs, extraction products obtained at these temperatures should be carefully analysed and studied.
increased from 66 °C to 200 °C. Variance However, at temperatures above 150 °C, rosmarinic acid and carnosic acid decreases (Table 2). As the AOA remained SourceIt is also important to note that a plant’s phenolic content itself may vary depending on plant growing conditions and plant genotypes [73]. Depending on the species, various forms of polyphenols and how they are bound to the plant tissue may vary and generate different effects on extraction temperature [57]. Heat treatment could aid in breaking the phenol–protein and phenol–polysaccharide bonds that increase extraction yield [22]. As the different phenolics in plant tissue are bound differently, the most effective method to extract the phenolic compounds will be different based on the plant species [17].
Palma et al. [36] performed a PLE for polyphenols from grape peels and seeds. They found that the temperature of extraction did not have a significant impact on the recovery rates from grape skin, but it did from the grape seed. As the breaking of bonds between the phenols and the plant matrix was facilitated at high temperatures, the authors sug- gested that the phenolic compounds in grape seed must have stronger bonds to the matrix compared with grape skin.
Barros et al. [50] studied the impact of PLE temperature on two types of sorghum brans and found that the optimum temperature was different for each type. As the profile of polyphenols within each type of sorghum bran is different, the optimal extraction temperature is also different. As seen in Table 2 above, the phenolic profile of each plant is very different, and due to this diversity, the hydrolysis that occurs during extractions at high temperatures may explain the variances reported in the literature.
To better understand the impact of the source’s plant matrix, we can specifically examine one well-studied source of polyphenols: honey. Many studies have investigated the impact of thermal processing on various types of honey, giving us insight into the behaviour of polyphenols.
Majkut et al. [80] found that among four nectar honey variants tested, all four showed an increase in TPC and AOA with thermal treatment at 100 ◦C. However, the extent to which it increased depended on the honey variant. For example, The TPC in rapeseed honey increased 15%, while a 27% increase was observed for buckwheat honey [80]. In contrast, Villacrés-Granda et al. [81] found that heat treatment at 60 ◦C caused a two-fold reduction in the TPC of eucalyptus honey.
Wang et al. [82] investigated the impact of thermal processing (82.2 ◦C for 10–12 s) on clover and buckwheat honey. There was no significant change in the TPC of clover honey, while the TPC in buckwheat honey showed a decrease. Another study [83] investigated the impact of thermal processing (90 ◦C up to 60 min) on honeydew, lime, acacia and buckwheat honey. The results, once again, varied depending on the origin of the honey. There was no significant change in TPC for acacia and buckwheat honey, while TPC increased for lime honey and decreased for honeydew honey [83]. Aydogan-Coskun, Coklar and Akbulut [84] compared the impact of liquefaction at 55 ◦C for 12 h and pasteurisation at 90 ◦C for 15 s on astragalus and sunflower–cornflower honey. They concluded that the variation in the impact of the process on TPC and AOA is based on the type of honey. Escriche et al. [85] studied the influence of heat treatments on phenolic profiles of citrus, rosemary, polyfloral and honeydew honey and also concluded that the flavonoids reacted differently to the heat treatments depending on the origin of the honey.
From the results of all the studies presented above, we can conclude that even within a single matrix (i.e., honey), the impact of temperature on polyphenols varies depending on the origin and type of honey.
7very high, the authors suggested that rosmarinic and carnosic acid broke down to compounds with higher antioxidant power.
To develop an understanding of the impact of temperature on the extraction of polyphe- nols, the extractants must be critically analysed. The total phenolic content (TPC) and antioxidant activity (AOA) are the most commonly used measures. The TPC is usually calculated using the Folin–Ciocâlteu assay or by HPLC analysis. The AOA can be calculated using a variety of assays: ABTS (2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid)), DPPH (2,2-diphenyl-1-picrylhydrazyl), FRAP (ferric-reducing antioxidant power or ORAC (oxygen radical absorption capacity) assays [86]. Most studies have performed more than one antioxidant assay to analyse the extractant.
- Overestimation of Total Phenolic Content by Folin–Ciocâlteu Assay
We need to develop a further understanding of how the TPC, as measured by the Folin–Ciocâlteu method, corresponds to the TPC measured by HPLC. It is difficult to measure TPC using HPLC, as a standard of each phenolic acid is needed to identify the peaks and quantify the area and, as a result, TPC calculated by HPLC is often much lower than that measured by the Folin–Ciocâlteu method [22,73].
As the Folin–Ciocâlteu method depends on the reducing power of phenolic hydroxyl
groups to estimate the TPC, it accounts for all the phenols and their degraded products. This lack of specificity often results in an overestimation of TPC.
According to Vergara-Salinas et al. [47] the TPC calculated by Folin–Ciocâlteu in-
creased with increasing temperature while the measurements of individual polyphenols and total polyphenols as calculated by HPLC suggested the opposite. The various polyphe- nols tested were hydroxyphenyl propanoic acids (HPPA), hydroxycinnamic acids, flavones, and flavanols/flavanones. Except for HPPA, the yield was highest at 100 ◦C and lowest at 200 ◦C for all compounds. HPPA and TPC calculated by the Folin–Ciocâlteu assay showed increased yield with increasing temperature. The total phenols calculated by the sum of areas of peaks in the HPLC chromatogram is lowest at 200 ◦C and highest at 100 ◦C, in contrast to the TPC measured by Folin–Ciocâlteu assay. At temperatures above 160 ◦C, water is able to solubilise even lignin and hemicellulose [47]. With the increase in solubility and hydrolytic reactions at high temperatures, these compounds may break down into phenolic acids, and the breakdown of lignocellulose may release not just phenolic materials but also reducing agents and sugars [54]. These additional compounds could also be detected by the Folin–Ciocâlteu assay causing further errors in the estimation of TPCs.
Mišan et al. [73] performed the quantification of phenolics (TPC) by HPLC and com- pared the result to the TPC values obtained by Folin–Ciocâlteu assay. The study included parsley, buckthorn, mint, caraway and birch extracts and found no significant correlation between the results of HPLC and the Folin–Ciocâlteu method. However, the difference between the results was not very high for parsley, mint and buckthorn. TPC in birch and parsley was overestimated by the Folin–Ciocâlteu method, while it underestimated caraway. This suggests the effectiveness of measuring TPC using the Folin assay varies depending on the source material.
Mandic et al. [87] found that the TPC and TFC calculated by the Folin–Ciocâlteu and HPLC methods is highly correlated (r = 0.90). However, the Folin–Ciocâlteu method resulted on average in a TPC 1.5–2.5 times higher than the HPLC results. Guendez et al. [88] found that TPC (HPLC) correlates to AOA with r2 = 0.628 and TPC (Folin–Ciocâlteu) correlates to AOA with r2 = 0.649. Once again, the TPC (HPLC) values are on average 2.9 times lower than TPC (Folin–Ciocâlteu) values, suggesting that other compounds present in the extracts that are accounted for in the TPC have little influence on the overall AOA.
- Lack of Consensus in the TPC Correlation to AOA
While most of the literature did find a strong correlation between TPC and AOA [19, 20,28,57,87–89], this is not always the case. There are quite a few publications that did not show such a correlation [43,46–48,90–92].
Budrat and Shotipruk [48] extracted polyphenols using PLE (130–200 ◦C) and methanol/ water extraction from bitter melon (65 ◦C). TPC increased with increasing temperature as did the specific polyphenols (catechin, gallic acid, gentisic acid and chlorogenic acid) that were studied, although gallic acid showed a slight decrease at 200 ◦C. As the AOA does not relate to TPC or the individual phenols here, the AOA must be exhibited by either phenolic compounds not tested for here or non-phenolic compounds such as vitamins and sugars that were decomposed at higher temperatures.
The highest AOA was observed at 150 ◦C and the lowest was at 200 ◦C, showing that higher extraction temperatures result in lower AOA. While the highest TPC was observed at 200 ◦C, it corresponds to the lowest AOA. However, even the lowest AOA observed at 200 ◦C in the PLE extraction is seven times higher than any of the traditional extractions performed (Soxhlet, methanol (at 65 ◦C) and water extraction (at 100 ◦C)).
Rodríguez-Meizoso et al. [46] performed a PLE extraction of polyphenols from dried oregano leaves at 25, 50, 100, 150 and 200 ◦C. The study showed that the temperatures did not significantly affect the TPC, although a drop was observed at 200 ◦C. The authors concluded that PLE does not, therefore, lead to the degradation or oxidation of phenolic compounds until 200 ◦C. However, the AOA increased at higher temperatures, suggesting that TPC is not correlated to AOA. The authors suggested that although the number of phenolic compounds is relatively constant, the variety and structure of the compounds may be changing in a way that increases the AOA.
The relationship between the structure of a polyphenol and its antioxidant capacity is not well understood. However, some studies have tried to establish a link between them. Benavente-García et al. [93] studied flavonoids in the citrus peels and established that ‘the antioxidant capacity of any flavonoid will be determined by a combination of the O-dihydroxy structure in the B-ring, the 2,3-double bond in conjugation with a 4-oxo function and the presence of both hydroxyl groups in positions 3 and 5t.
8. phenols occurs at high temperatures, researchers can compare the results of this study to other papers CperfonclusionsTo understand the behaviour of polyphenols during extraction at high temperatures, one needs to understand all the factors that affect this parameter. Thermal degradation of different types of polyphenols occurs at different temperatures, but it depends on the pre-treatment, solvent type, pH, treatment time, extraction environment and source of the material. The extraction temperature has a significant effect on the types of polyphenols being extracted. Further studies are needed to understand the role of specific phenolic acids and their antioxidant activity. The formation of new compounds (MRPs) at the high temperatures under which PLE is performed should also be investigated.
The lack of specificity of the Folin–Ciocâlteu assay for calculating TPC and the lack
of understanding on how TPC relates to AOA makes it very difficult to establish a clear understanding of the reported conflicting effect of temperature on polyphenols. It is recommended that HPLC analysis of various phenolic acids should be performed along with TPC by Folin–Ciocâlteu assay to develop a better understanding of the extracts phenolic profile. The review concludes that thermal degradation alone does not explain the decrease in phenolic yield at temperatures above 90 ◦C, and all the factors discussed in the paper should be taken into account to understand the effect of temperature on polyphenols.
Rrming extractions at high temperatures. The results show that depending on the plant, many polyphenols appear or disappear aferter hydrolysis. Table 2 below summarises studies that showed a shiftinces- Pandey, B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease.
- Oxid. Med. Cell. Longev.
- 2009
- ,
- 2
- , 270–278. [
- ] [
- ]
- Zia-Ul-Haq, Historical and Introductory Aspects of Carotenoids. In
- Carotenoids: Structure and Function in the Human Body
- ; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–42. ISBN 978-3-030-46459-2.
- Cory, ; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review.
Front. Nutr. 2018, 5, 87. [CrossRef] [PubMed]
- Arts, C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [CrossRef] [PubMed]
- Todd, ; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [CrossRef]
- Nayak, ; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag.
2019, 233, 352–370. [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. Food Eng. 2013, 117, 426–436. [CrossRef]
- Galanakis, M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [CrossRef]
- Soquetta, B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables.
CYTA J. Food 2018, 16, 400–412. [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Food Eng. Rev. 2016, 8, 23–34. [CrossRef]
- Dent, ; Dragovic´-Uzelac, V.; Penic´, M.; Brñic´, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91.
- Bucic´-Kojic´, ; Planinic´, M.; Tomas, S.; Bilic´, M.; Velic´, D. Study of solid-liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007, 81, 236–242. [CrossRef]
- Jokic, ; Velic, D.; Bilic, M.; Bucic-Kojic, A.; Planinic, M.; Tomasa, S. Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech J. Food Sci. 2010, 28, 206–212. [CrossRef]
- Durling, E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [CrossRef]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. Food Sci. 2003, 68, 240–248. [CrossRef]
- Jeong, M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004, 52, 3389–3393. [CrossRef]
- Spigno, ; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [CrossRef]
- Akowuah, A.; Mariam, A.; Chin, J.H. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn. Mag. 2009, 4, 81–85.
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. Food Process. Preserv. 2016, 40, 103–115. [CrossRef]
- Li, B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006,
48, 182–188. [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol J. Food Agric. Environ. 2003, 1, 42–47.
- Jeganathan, P.M.; Venkatachalam, S.; Karichappan, T.; Ramasamy, S. Model development and process optimization for solvent extraction of polyphenols from red grapes using box-behnken design. Biochem. Biotechnol. 2014, 44, 56–67. [CrossRef] [PubMed]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Eng. Res. Des. 2011, 89, 857–862. [CrossRef]
- Cˇepo, V.; Albahari, P.; Koncˇic´, M.Z.; Radic´, K.; Jurmanovic´, S.; Jug, M. Solvent Extraction and Chromatographic Determination
of Polyphenols in Olive Pomace. Food Health Dis. 2017, 6, 7–14.
- Rajbhar, ; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Rev. Chem. Commun 2015, 5, 1–6.
- Bucic´-Kojic´, ; Planinic´, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401. [CrossRef]
- Larrauri, A.; Sánchez-Moreno, C.; Saura-Calixto, F. Effect of Temperature on the Free Radical Scavenging Capacity of Extracts from Red and White Grape Pomace Peels. J. Agric. Food Chem. 1998, 46, 2694–2697. [CrossRef]
- Al Juhaimi, ; Özcan, M.M.; Uslu, N.; Ghafoor, K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018, 55, 190–197. [CrossRef]
- Lang, H.; Lindemann, I.D.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204. [CrossRef]
- Daniel, L.; Huerta, B.E.B.; Sosa, I.A.; Mendoza, M.G.V. Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Ind. Crops Prod. 2012, 40, 268–276. [CrossRef]
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, var. Hungarian). Food Chem. 2009, 117, 647–653. [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa ). Ind. Crops Prod. 2010, 32, 258–263. [CrossRef]
- Ross, F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890. [CrossRef] [PubMed]
- Larrauri, A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [CrossRef]
- Palma, ; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174. [CrossRef]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, U.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. Agric. Food Chem. 2003, 51, 375–382. [CrossRef]
- Zekovic´, ; Vidovic´, S.; Vladic´, J.; Radosavljevic´, R.; Cvejin, A.; Elgndi, M.A.; Pavlic´, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J. Supercrit. Fluids 2014, 95, 560–566. [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape Int. J. Food Sci. Technol. 2012, 47, 399–405. [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba J. Food Eng. 2012, 108, 444–452. [CrossRef]
- Howard, ; Pandjaitan, N. Pressurized liquid extraction of flavonoids from spinach. J. Food Sci. 2008, 73. [CrossRef]
- Andrew, J.; Masetlwa, J.; Tesfaye, T.; Sithole, B. Beneficiation of eucalyptus tree barks in the context of an integrated biorefinery— Optimisation of accelerated solvent extraction (ASE) of polyphenolic compounds using response surface methodology. Chem. Pharm. 2020, 18, 100327. [CrossRef]
- Ollanketo, ; Peltoketo, A.; Hartonen, K.; Hiltunen, R.; Riekkola, M.L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: Antioxidant activity of the extracts. Eur. Food Res. Technol. 2002, 215, 158–163. [CrossRef]
- Nandasiri, ; Eskin, N.A.M.; Thiyam-Höllander, U. Antioxidative Polyphenols of Canola Meal Extracted by High Pressure: Impact of Temperature and Solvents. J. Food Sci. 2019, 84, 3117–3128. [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis ), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [CrossRef]
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. Pharm. Biomed. Anal. 2006, 41, 1560–1565. [CrossRef]
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). Agric. Food Chem. 2012, 60, 10920–10929. [CrossRef]
- Budrat, P.; Shotipruk, A. Enhanced recovery of phenolic compounds from bitter melon (Momordica charantia) by subcritical water Sep. Purif. Technol. 2009, 66, 125–129. [CrossRef]
- Repajic´, ; Cegledi, E.; Kruk, V.; Pedisic´, S.; Çinar, F.; Kovacˇevic´, D.B.; Žutic´, I.; Dragovic´-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments fromwild nettle leaves. Processes 2020, 8, 803. [CrossRef]
- Barros, F.; Dykes, L.; Awika, J.M.; Rooney, L.W. Accelerated solvent extraction of phenolic compounds from sorghum brans. Cereal Sci. 2013, 58, 305–312. [CrossRef]
- Álvarez-Casas, M.; García-Jares, C.; Llompart, M.; Lores, M. Effect of experimental parameters in the pressurized solvent extraction of polyphenolic compounds from white grape Food Chem. 2014, 157, 524–532. [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the drying process on the intensification of phenolic compounds recovery from grape pomace using accelerated solvent extraction. J. Mol. Sci. 2014, 15, 18640–18658. [CrossRef] [PubMed]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and Food Bioprod. Process. 2015, 94, 29–38. [CrossRef]
- Maillard, N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [CrossRef]
- Mokrani, ; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [CrossRef]
- Chen, L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185. [CrossRef]
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed Ultrason. Sonochem. 2014, 21, 346–353. [CrossRef] [PubMed]
- Dorta, ; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [CrossRef]
- Wang, F.; Kim, D.M.; Lee, C.Y. Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J. Agric. Food Chem. 2000, 48, 4227–4232. [CrossRef] [PubMed]
- Patras, ; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [CrossRef]
- Alvarez-Suarez, M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramás, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research—Plant fortification and bioavailability issues. Trends Food Sci. Technol. 2021, 117, 92–105. [CrossRef]
- Ju, Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [CrossRef]
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605. [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. Food Sci. 2006, 71, C504–C512. [CrossRef]
- Havlíková, ; Míková, K. Heat Stability of Anthocyanins. Z. Lebensm. Unters. Forsch. 1985, 181, 427–432. [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger Food Chem. 2009, 113, 166–172. [CrossRef]
- Tran, T.M.K.; Kirkman, T.; Nguyen, M.; Van Vuong, Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6, [CrossRef]
- Valadez-Carmona, ; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea- Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [CrossRef]
- Kader, F.; Irmouli, M.; Nicolas, J.P.; Metche, M. Involvement of blueberry peroxidase in the mechanisms of anthocyanin degradation in blueberry J. Food Sci. 2002, 67, 910–915. [CrossRef]
- Vamos-Vigyázó, Polyphenol Oxidase and Peroxidase in Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127.
[CrossRef]
- Rossi, ; Giussani, E.; Morelli, R.; Lo Scalzo, R.; Nanic, R.C.; Torreggiani, D. Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Res. Int. 2003, 36, 999–1005. [CrossRef]
- Skrede, ; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364. [CrossRef]
- Mišan, Cˇ.; Mimica-Dukic´, N.M.; Mandic´, A.I.; Sakacˇ, M.B.; Milovanovic´, I.L.; Sedej, I.J. Development of a Rapid Resolution
HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent. Eur. J. Chem. 2011, 9, 133–142. [CrossRef]
- Jablonský, ; Vernarecová, M.; Ház, A.; Dubinyová, L.; Škulcová, A.; Sladková, A.; Šurina, I. Extraction of phenolic and lipophilic compounds from spruce (picea abies) bark using accelerated solvent extraction by ethanol. Wood Res. 2015, 60, 583–590.
- Mustafa, A.; Turner, Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [CrossRef] [PubMed]
- Arvidsson, ; van Boekel, M.A.J.S.; Skog, K.; Jägerstad, M. Formation of Mutagenic Maillard Reaction Products. Mail. React. Foods Med. 2005, 219–224. [CrossRef]
- Kim, S.; Lee, Y.S. Characteristics and antioxidant activity of Maillard reaction products from fructose-glycine oligomer. Food Sci. Biotechnol. 2010, 19, 929–940. [CrossRef]
- Plaza, ; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010, 43, 1123–1129. [CrossRef]
- Lim, Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT Food Sci. Technol. 2007, 40, 1664–1669. [CrossRef]
- Majkut, ; Kwiecin´ska-Piróg, J.; Wszelaczyn´ska, E.; Poberez˙ny, J.; Gospodarek-Komkowska, E.; Wojtacki, K.; Barczak, T. Antimicrobial activity of heat-treated Polish honeys. Food Chem. 2021, 343, 128561. [CrossRef]
- Villacrés-Granda, I.; Proaño, A.; Coello, D.; Debut, A.; Vizuete, K.; Ballesteros, I.; Granda-Albuja, G.; Rosero-Mayanquer, H.; Battino, M.; Giampieri, F.; et al. Effect of thermal liquefaction on quality, chemical composition and antibiofilm activity against multiresistant human pathogens of crystallized eucalyptus Food Chem. 2021, 365, 130519. [CrossRef]
- Wang, H.; Gheldof, N.; Engeseth, N.J. Effect of Processing and Storage on Antioxidant Capacity of Honey. J. Food Sci. 2004, 69,
96–101. [CrossRef]
- Kowalski, Changes of antioxidant activity and formation of 5-hydroxymethylfurfural in honey during thermal and microwave processing. Food Chem. 2013, 141, 1378–1382. [CrossRef]
- Aydogan-Coskun, ; Coklar, H.; Akbulut, M. Effect of heat treatment for liquefaction and pasteurization on antioxidant activity anphenolic compounds of astragalus and sunflower-cornflower honeys. Food Sci. Technol. 2020, 40, 629–634. [CrossRef]
- Escriche, ; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142, 135–143. [CrossRef]
- Thaipong, ; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [CrossRef]
- Mandic, I.; Dilas, S.M.; C´etkovic´, G.S.; Cˇ anadanovic´-Brunet, J.M.; Tumbas, V.T. Polyphenolic composition and antioxidant
activities of grape seed extract. Int. J. Food Prop. 2008, 11, 713–726. [CrossRef]
- Guendez, ; Kallithraka, S.; Makris, D.P.; Kefalas, P. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: Correlation with antiradical activity. Food Chem. 2005, 89, 1–9. [CrossRef]
- Ferguson, R. Kiwifruit: The Wild and the Cultivated Plants. In Advances in Food and Nutrition Research; Boland, M., Moughan,
P.J., Eds.; Academic Press Inc.: Waltham, MA, USA, 2013; Volume 68, pp. 15–32, ISBN 9780123942944.
- Heinonen, M.; Lehtonen, P.J.; Hopia, A.I. Antioxidant Activity of Berry and Fruit Wines and Liquors. J. Agric. Food Chem. 1998,
46, 25–31. [CrossRef]
- Kähkönen, P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [CrossRef]
- Lafka, I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes.
Food Chem. 2007, 104, 1206–1214. [CrossRef]
- Benavente-García, ; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and Properties of Citrus Flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [CrossRef]
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Zia-Ul-Haq, M. Historical and Introductory Aspects of Carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–42. ISBN 978-3-030-46459-2.
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87.
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S.
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358.
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436.
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87.
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA J. Food 2018, 16, 400–412.
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2016, 8, 23–34.
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brñić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91.
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velić, D. Study of solid-liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007, 81, 236–242.
- Jokic, S.; Velic, D.; Bilic, M.; Bucic-Kojic, A.; Planinic, M.; Tomasa, S. Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech J. Food Sci. 2010, 28, 206–212.
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424.
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295.
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248.
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004, 52, 3389–3393.
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208.
- Akowuah, G.A.; Mariam, A.; Chin, J.H. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn. Mag. 2009, 4, 81–85.
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit By-Products: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2016, 40, 103–115.
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188.
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47.
- Jeganathan, P.M.; Venkatachalam, S.; Karichappan, T.; Ramasamy, S. Model development and process optimization for solvent extraction of polyphenols from red grapes using box-behnken design. Prep. Biochem. Biotechnol. 2014, 44, 56–67.
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862.
- Čepo, D.V.; Albahari, P.; Končić, M.Z.; Radić, K.; Jurmanović, S.; Jug, M. Solvent Extraction and Chromatographic Determination of Polyphenols in Olive Pomace. Food Health Dis. 2017, 6, 7–14.
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Rev. Chem. Commun 2015, 5, 1–6.
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Jakobek, L.; Šeruga, M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int. J. Food Sci. Technol. 2009, 44, 2394–2401.
- Larrauri, J.A.; Sánchez-Moreno, C.; Saura-Calixto, F. Effect of Temperature on the Free Radical Scavenging Capacity of Extracts from Red and White Grape Pomace Peels. J. Agric. Food Chem. 1998, 46, 2694–2697.
- Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Ghafoor, K. The effect of drying temperatures on antioxidant activity, phenolic compounds, fatty acid composition and tocopherol contents in citrus seed and oils. J. Food Sci. Technol. 2018, 55, 190–197.
- Lang, G.H.; Lindemann, I.D.S.; Ferreira, C.D.; Hoffmann, J.F.; Vanier, N.L.; de Oliveira, M. Effects of drying temperature and long-term storage conditions on black rice phenolic compounds. Food Chem. 2019, 287, 197–204.
- Daniel, D.L.; Huerta, B.E.B.; Sosa, I.A.; Mendoza, M.G.V. Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.). Ind. Crops Prod. 2012, 40, 268–276.
- Vega-Gálvez, A.; Di Scala, K.; Rodríguez, K.; Lemus-Mondaca, R.; Miranda, M.; López, J.; Perez-Won, M. Effect of air-drying temperature on physico-chemical properties, antioxidant capacity, colour and total phenolic content of red pepper (Capsicum annuum, L. var. Hungarian). Food Chem. 2009, 117, 647–653.
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind. Crops Prod. 2010, 32, 258–263.
- Ross, C.F.; Hoye, C.; Fernandez-Plotka, V.C. Influence of Heating on the Polyphenolic Content and Antioxidant Activity of Grape Seed Flour. J. Food Sci. 2011, 76, 884–890.
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. J. Agric. Food Chem. 1997, 45, 1390–1393.
- Palma, M.; Piñeiro, Z.; Barroso, C.G. Stability of phenolic compounds during extraction with superheated solvents. J. Chromatogr. A 2001, 921, 169–174.
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, U.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. J. Agric. Food Chem. 2003, 51, 375–382.
- Zeković, Z.; Vidović, S.; Vladić, J.; Radosavljević, R.; Cvejin, A.; Elgndi, M.A.; Pavlić, B. Optimization of subcritical water extraction of antioxidants from Coriandrum sativum seeds by response surface methodology. J. Supercrit. Fluids 2014, 95, 560–566.
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405.
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452.
- Howard, L.; Pandjaitan, N. Pressurized liquid extraction of flavonoids from spinach. J. Food Sci. 2008, 73.
- Andrew, J.; Masetlwa, J.; Tesfaye, T.; Sithole, B. Beneficiation of eucalyptus tree barks in the context of an integrated biorefinery—Optimisation of accelerated solvent extraction (ASE) of polyphenolic compounds using response surface methodology. Sustain. Chem. Pharm. 2020, 18, 100327.
- Ollanketo, M.; Peltoketo, A.; Hartonen, K.; Hiltunen, R.; Riekkola, M.L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: Antioxidant activity of the extracts. Eur. Food Res. Technol. 2002, 215, 158–163.
- Nandasiri, R.; Eskin, N.A.M.; Thiyam-Höllander, U. Antioxidative Polyphenols of Canola Meal Extracted by High Pressure: Impact of Temperature and Solvents. J. Food Sci. 2019, 84, 3117–3128.
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346.
- Rodríguez-Meizoso, I.; Marin, F.R.; Herrero, M.; Señorans, F.J.; Reglero, G.; Cifuentes, A.; Ibáñez, E. Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J. Pharm. Biomed. Anal. 2006, 41, 1560–1565.
- Vergara-Salinas, J.R.; Pérez-Jiménez, J.; Torres, J.L.; Agosin, E.; Pérez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929.
- Budrat, P.; Shotipruk, A. Enhanced recovery of phenolic compounds from bitter melon (Momordica charantia) by subcritical water extraction. Sep. Purif. Technol. 2009, 66, 125–129.
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çinar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments fromwild nettle leaves. Processes 2020, 8, 803.
- Barros, F.; Dykes, L.; Awika, J.M.; Rooney, L.W. Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci. 2013, 58, 305–312.
- Álvarez-Casas, M.; García-Jares, C.; Llompart, M.; Lores, M. Effect of experimental parameters in the pressurized solvent extraction of polyphenolic compounds from white grape marc. Food Chem. 2014, 157, 524–532.
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the drying process on the intensification of phenolic compounds recovery from grape pomace using accelerated solvent extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658.
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38.
- Maillard, M.N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793.
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76.
- Chen, M.L.; Yang, D.J.; Liu, S.C. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Technol. 2011, 46, 1179–1185.
- Teh, S.S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353.
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88.
- Wang, L.F.; Kim, D.M.; Lee, C.Y. Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J. Agric. Food Chem. 2000, 48, 4227–4232.
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11.
- Alvarez-Suarez, J.M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramás, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research—Plant fortification and bioavailability issues. Trends Food Sci. Technol. 2021, 117, 92–105.
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213.
- Volden, J.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.; Thygesen, I.E.; Wicklund, T. Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem. 2008, 109, 595–605.
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006, 71, C504–C512.
- Havlíková, L.; Míková, K. Heat Stability of Anthocyanins. Z. Lebensm. Unters. Forsch. 1985, 181, 427–432.
- Chan, E.W.C.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172.
- Tran, T.M.K.; Kirkman, T.; Nguyen, M.; Van Vuong, Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6, e04498.
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386.
- Kader, F.; Irmouli, M.; Nicolas, J.P.; Metche, M. Involvement of blueberry peroxidase in the mechanisms of anthocyanin degradation in blueberry juice. J. Food Sci. 2002, 67, 910–915.
- Vamos-Vigyázó, L. Polyphenol Oxidase and Peroxidase in Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127.
- Rossi, M.; Giussani, E.; Morelli, R.; Lo Scalzo, R.; Nanic, R.C.; Torreggiani, D. Effect of fruit blanching on phenolics and radical scavenging activity of highbush blueberry juice. Food Res. Int. 2003, 36, 999–1005.
- Skrede, G.; Wrolstad, R.E.; Durst, R.W. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J. Food Sci. 2000, 65, 357–364.
- Mišan, A.Č.; Mimica-Dukić, N.M.; Mandić, A.I.; Sakač, M.B.; Milovanović, I.L.; Sedej, I.J. Development of a Rapid Resolution HPLC method for the separation and determination of 17 phenolic compounds in crude plant extracts. Cent. Eur. J. Chem. 2011, 9, 133–142.
- Jablonský, M.; Vernarecová, M.; Ház, A.; Dubinyová, L.; Škulcová, A.; Sladková, A.; Šurina, I. Extraction of phenolic and lipophilic compounds from spruce (picea abies) bark using accelerated solvent extraction by ethanol. Wood Res. 2015, 60, 583–590.
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18.
