Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Camila Xu and Version 2 by Camila Xu.
African swine fever virus (ASFV) is the causative agent of the epidemic of African swine fever (ASF), with virulent strains having a mortality rate of up to 100% and presenting devastating impacts on animal farming. ASFV is the only known arbovirus in the Nucleoplasmic Large DNA Virus (NCLDV) family. It has an icosahedral structure and envelope with a diameter of 200nm. The length of the genome varies between 170 and 190 kbp, encoding 151 to 167 open reading frames (ORF), which are closely spaced along two strands of viral DNA and separated by short intergenic regions.
- African swine fever virus
- immune escape
- immune response
1. Introduction
In 1921, African swine fever virus (ASFV) was first reported in Kenya, which caused about an 100% mortality in infected domestic pigs [1]. Subsequently, ASFV spread to most African countries south of the Sahara [2]. In 1957 and 1960, the African swine fever epidemic broke out in Portugal which was the first time to demonstrate that ASFV spread across continents and spread to other European countries, the Caribbean, and Brazil [3]. Except in Sardinia, the genotype I ASFV was eradicated in the 1990’s [4]. However, the genotype II ASFV was introduced to Georgia in the Caucasus region and started a new round of transmission in 2007 [5]. Afterwards, it spread to the Russian Federation, Ukraine and Belarus, and in 2014 it spread to Eastern European countries [4,5][4][5]. In 2018, the epidemic spread to Belgium, Hungary, Czech Republic, Romania, Bulgaria, Slovakia, and Serbia, as well as China and other parts of Asia (Mongolia, Korea, Vietnam, Laos, Cambodia, Myanmar, the Philippines, Hong Kong, and Indonesia) [3]. ASF outbreaks are still trending regionally, with new regional reports of ASF outbreaks. In September 2019, Timor-Leste reported the first outbreak of African swine fever in Oceania, followed by Papua New Guinea (March 2020) [6,7][6][7]. In July 2021, ASF reappeared in the Americas, first in the Dominican Republic and later in Haiti [8,9][8][9]. In January 2022, ASF genotype II was notified in mainland Italy [9]. In January 2022, two new countries also reported their first cases of the disease: North Macedonia in Europe and Thailand in Asia [9]. According to OIE, from January 2020 to January 2022, ASF outbreaks were reported in 35 countries or regions around the world, including 4767 cases (1043334 animals lost) in domestic pigs and 18,262 cases (29970 animals lost) in wild boars [9]. It is worth noting that wild boar cases in Europe accounted for the vast majority (83.3%, 16743/20107), while wild boar cases in Asia accounted for a lower proportion of 59.4% (1519/2559) [9]. The wild boar reservoir of ASFV will present challenges to the eradication of ASF by vaccination programs.
In August 2018, the first ASF case in China was identified in Shenyang, Liaoning Province [10]. Since most pig farms in China are small-scale pig farms with very low biosecurity levels, the rapid spread of ASFV across the country has led to a serious reduction in the number of live pigs and caused heavy losses to the pig industry [11]. The decline in the number of live pigs has led to a rapid rise in the price of pork in a short period of time, affecting the normal life of residents in China. After the outbreak of ASF, with the joint efforts of Chinese government leaders and industry practitioners, various pig farms tried to resume production. By improving the level of biosecurity, including optimizing the location of pig farms, strict disinfection measures, and strengthening ASFV testing on pig farms, ASFV-positive pigs were culled at designated locations, and the source of the infection was eliminated. Most pig farms finally successfully resumed breeding [12]. However, in order to improve the level of biosecurity, the measures taken by pig farms have objectively increased the cost of breeding. Therefore, there is an urgent need for vaccines for the effective control and eradication of ASF.
Hemadsorption (HAD) refers to the ability of ASFV-infected macrophages to adsorb erythrocytes, and non-hemadsorption (non-HAD) refers to the inability of ASFV-infected macrophages to adsorb erythrocytes. ASFV has spread for more than three years in China, and its epidemic trend has become more complicated with the emergence of non-hemadsorbing (non-HAD) naturally attenuated strains of ASFV [13,14,15][13][14][15]. The initial clinical symptoms of domestic pigs infected with low-viral strains are not obvious, which is difficult to diagnose [13,14][13][14]. Comparative analysis showed that the genome of the low-virulence strains had mutations, deletions, insertions or substitutions of short fragments, including the deletion or mutation of the CD2v (EP402R) gene, which is related to the HAD phenotype [16]. In addition, genotype I strains have also been reported in China (Figure 1) [14]. The potential for endemic ASF outbreaks is increasing due to the widespread distribution of wild boars in China. Researchers are actively conducting ASF vaccine-related research, including the evaluation of live attenuated vaccines (LAVs) vaccines by knocking out virulence genes, and the screening of antigens with protective effects to develop subunit vaccines, vector vaccines, etc. [17,18,19,20,21,22,23,24,25,26,27,28,29,30][17][18][19][20][21][22][23][24][25][26][27][28][29][30]. ASFV has evolved the ability to manipulate host immune responses by encoding many immune escape genes, including regulation of IFN expression, inhibition of autophagy, and apoptosis. In addition, ASFV affects the antigen presentation and activation of lymphocytes by invading monocytes-macrophages and DCs, thereby affecting the immune system of the entire body [31].
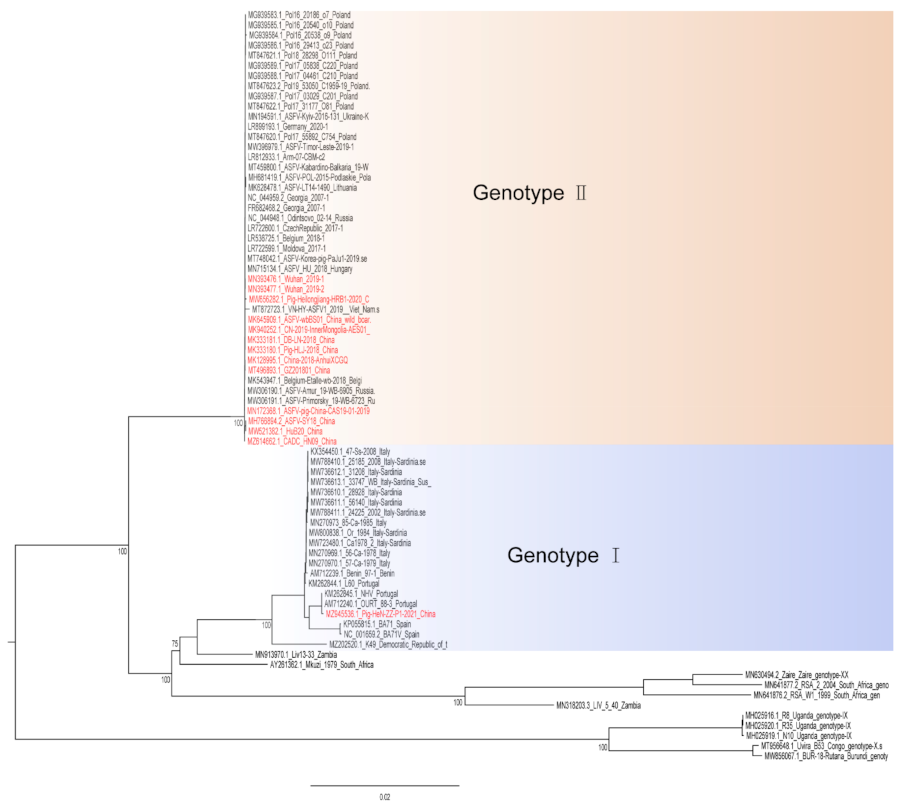
Figure 1. A phylogenetic tree was constructed based on the whole genome sequences of 74 strains in the GenBank database, after which datasets for the sequences were aligned using MAFFT (version 7.149) program [32]. Maximum likelihood (ML) phylogenies for the codon alignment of the genome sequences were estimated using the GTRGAMMA nucleotide substitution model in the IQ-TREE 1.68 software [33]. Node support was determined by nonparametric bootstrapping with 1000 replicates, and the phylogenetic tree was visualized in the Figtree (version 1.4.3) program (http://tree.bio.ed.ac.uk/software/Figtree/) (accessed on 18 January 2022). Types written in red indicate Chinese isolates.
2. The Major Defense Mechanisms and African Swine Fever Virus (ASFV) Interactions
2.1. Physical Barriers
The skin barrier is the most effective physical defense of the host to block entry of pathogens. The physical barriers of the body’s defenses include intact skin, surfaces of the respiratory and gastrointestinal tracts and include processes of self-cleaning (coughing, sneezing and mucus flow in the respiratory tract, vomiting, diarrhea, and urination of the urinary system, etc.), and normal flora on the skin surface and in the gut. The transmission of ASFV among pigs is mainly direct contact transmission, including transmission through mouth-nose contact and short-distance aerosol transmission [34]. In addition, the presence of soft tick bites in pig farms can also cause the transmission of ASFV [35]. Therefore, it is necessary to improve animal welfare in the farm, and increase the resistance of animals by reducing the breeding density. Pig farms should strengthen environmental sanitation and disinfection to block the spread of the virus. On the contrary, for infected animals, the self-cleaning of the body is also conducive to virus spread. It is necessary to regularly collect and detect the presence of ASFV in pig herds, and to ensure the removal and safe destruction of positive pig herds to eliminate the source of infection [12].
2.2. Innate Immunity
Sentinel cells, such as macrophages, dendritic cells, and mast cells, elicit inflammatory responses by recognizing pathogen-associated molecular patterns (PAMPs) of microorganisms through pattern recognition receptors. The inflammatory response involves the activation and directed migration of various cells from the blood to the site of invasion, especially neutrophils and macrophages. The pattern recognition receptors that recognize ASFV mainly include TLR3 that recognizes viral dsRNA and cGAS-STING that recognizes viral DNA in the cytoplasm [36,37,38][36][37][38]. When TLR3 binds to dsRNA, it transmits signals to cells, resulting in increased expression of nuclear factor kappa-B (NF-κB), which in turn activates IL-1, IL-6 and TNF-α and other cytokine expression [36]. The cGAS-STING system recognizes virus DNA, and the virus-infected (mainly plasmacytoid dendritic cells) cells produce IFN-I [38]. IFN-I acts on virus-infected cells to inhibit virus growth.
ASFV has also acquired many genes in evolution that can regulate the inflammatory response in the early stage of the body. ASFV-encoded genes also primarily inhibit the actions of TLR3 and the cGAS-STING system (Figure 2). Studies have shown that the I329L and A276R proteins encoded by ASFV have immunosuppressive effects [39,40][39][40]. I329L (the homologous protein of TLR3) interacts with the adaptor protein TRIF to inhibit the activation of NF-κB and IRF3, while A276R can also inhibit the recognition of TLR3 [37,39][37][39]. In addition, another study showed that TLR expression was decreased after ASFV infection of macrophages [40].
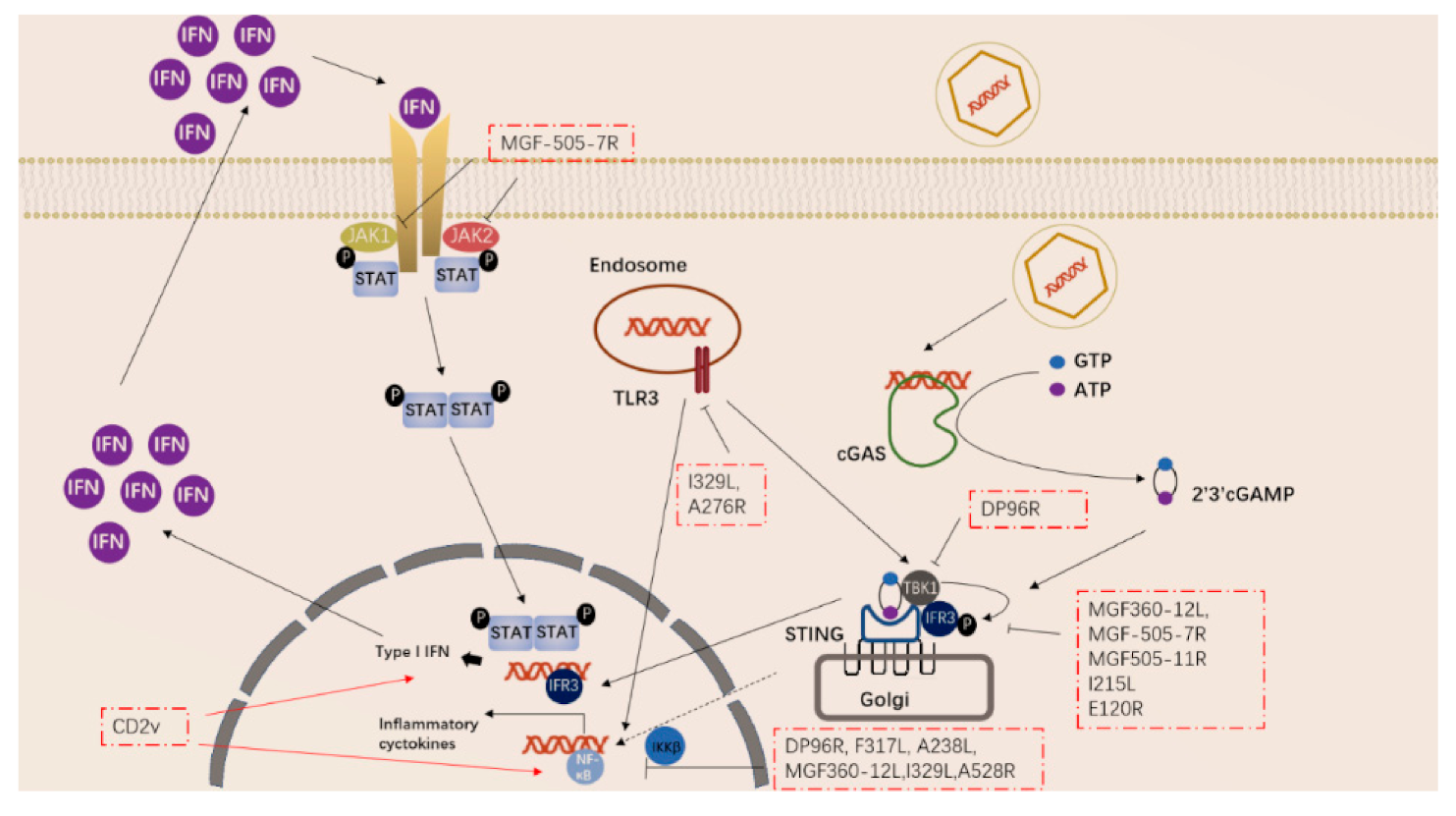

Figure 2. ASFV genes regulate innate immune signaling pathway.
ASFV encodes multiple genes involved in suppressing the cGAS-STING signaling pathway. In 2013, Correia et al. reported that A528R (MGF530) could inhibit the expression of IFN-β by targeting IRF3 and NF-κB or through the JAK-STAT pathway [39,41][39][41]. In 2018, Wang et al. found that DP96R targets TBK1 and IκB kinase beta (IKKβ) to negatively regulate IFN-I expression and induction of NF-κB signaling [42]. In 2020, Zhuo et al. demonstrated that MGF360-12L can block the interaction between p65 and KPNA2, KPNA3, and KPNA4, and can interfere with the nuclear translocation of NF-κB [43]. Five immunosuppressive genes were reported in 2021, including E120R, F317L, MGF505-7R, MGF505-11R, and I215L [44,45,46,47,48,49][44][45][46][47][48][49]. E120R can interact and block the activation of IRF3, thereby inhibiting the expression of IFN-β [44]. F317L inhibits IKKβ phosphorylation, thereby reducing the phosphorylation and ubiquitination of IkBα, thereby inhibiting the activation of the NF-κB pathway [45]. Li et al. reported that MGF-505-7R promoted the expression of autophagy-related protein ULK1 to degrade STING, and inhibited IFN-γ-mediated JAK1 and JAK2-mediated signaling pathways [46,48][46][48]. Similarly, Yang et al. reported that MGF505-11R interacts with STING, degrades STING expression through lysosome, ubiquitin-proteasome and autophagy pathways, and can inhibit the phosphorylation of TBK1 and IRF3 stimulated by cGAS/STING overexpression [47]. pI215L recruits RNF138 to inhibit K63-related TBK1 ubiquitination, which has an inhibitory effect on IFN-I production [49]. It is worth noting that CD2V can activate NF-κB and induce activation of IFN signaling pathways in porcine lymphocytes/macrophages [50]. These immunosuppressive genes are potential target genes for the preparation of live attenuated vaccines.
3. ASF Vaccines
3.1. Live Attenuated Vaccines (LAVs)
LAVs can generally be divided into natural attenuated strains and artificial gene deletion strains, and the latter is made by deleting certain virulence genes. Compared with other types of ASF vaccines, LAVs can provide complete homologous and partial heterologous protection [79][51]. The most promising LAVs vaccine candidates are summarized in Table 1. Chen et al. reported that a seven-gene deletion LAV had a good protective effect [17]. In addition, Zhang et al. reported that deleting L7L-L11L attenuates ASFV. In vaccination experiments, these attenuated strains conferred 100% protection against homologous challenge [18]. LAVs have experienced a trend from multiple gene deletions to single gene deletions. Protection induced by immunization with LAVs correlates with the level of replication of the LAVs in addition to the number of immunogenic genes expressed. If the replication of LAVs is severely attenuated, then the LAVs will not be immunogenic. LAVs with single gene deletions express more genes and may cause better protection. ASFV-G-∆I177L could achieve 100% protection through oral and injection routes, and field trials have been completed in Vietnam [19,20,21][19][20][21]. Similarly, ASFV-G-ΔA137R and SY18ΔI226R were proven to confer 100% protection [22,23][22][23].
Table 1. Summary of the most promising LAV candidates.
| Genes | Strains | Genotype | Minimal Protective Dose | Route | Challenge | Gene Function | References |
|---|---|---|---|---|---|---|---|
| I177L | Georgia2007/1 | II | 102HAD50 | IM | Georgia2007/1 | unknown | [19] |
| 106HAD50 | ON | Georgia2007/1 | [20] | ||||
| 102HAD50 | IM | TTKN/ASFV/DN/2019 | [21] | ||||
| A137R | Georgia2007/1 | II | 102HAD50 | IM | Georgia2007/1 | unknown | [23] |
| I226R I226R |
SY18 | II | 104HAD50 | IM | SY18 | unknown | [22] |
| L7L-L11L | SY18 | II | 103HAD50 | IM | SY18 | unknown | [18] |
| MGF505/360(6) 1 and EP402R | HLJ/18 | II | 103HAD50 | IM | HLJ/18 | hemadsorbing and inhibition of type I interferon responses | [17] |
| 105HAD50 | ON | ||||||
| EP402R | Ba71V | I | 104HAD50 | IM | Ba71V | hemadsorbing | [86][52] |
| E75 | |||||||
| Georgia2007/1 |
1: MGF505-1R, MGF505-2R, MGF505-3R, MGF360-12L, MGF360-13L, MGF360-14L.
Historically, attenuated strains were once used in Spain and Portugal, but widely caused chronic ASF infections in vaccinated pigs. Therefore, adequate experiments should be conducted to verify the effectiveness and safety of LAVs before extensive promotion, for example, future ASF vaccines should be distinguish between infected animals and vaccinated animals (DIVA) [80][53]. Ramirez-Medina et al. constructed a deletion strain of E184L to distinguish between infected animals and vaccinated animals (DIVA), but the deletion strain cannot provide complete protection [80][53]. LAVs are still far from commercialization due to the safety issue. LAVs may have the risk of virulence reversion during large-scale vaccination. Therefore, adequate experiments should be conducted to verify the effectiveness and safety of LAVs before extensive promotion.
In addition, the lack of cell lines for large-scale LAVs production is another difficulty. ASFV subculture can adapt to cell lines such as 293 and Vero, but the virulence and antigenicity of the adapted strains are weakened, and adapted strains cannot provide effective protection after immunizing pigs [81,82][54][55]. The adapted cells cultured in LAVs are primary cells, mainly including porcine alveolar macrophages (PAM) and porcine bone marrow cells (PBMS). Primary cells are not sufficient for large-scale production of LAVs. Borca et al. reported that ASFV-G-ΔI177L/ΔLVR could replicate efficiently in stable porcine cell lines with potent protection [83][56]. In addition, Takenouchi et al. reported a novel immortalized porcine macrophage cell line, IPKM, which was generated by transduction of primary PKM with lentiviral vectors encoding SV40LT and pTERT [84][57]. However, it is necessary to carefully test it before using IPKM as a production cell line for a live-attenuated vaccine strain.
Finally, the cross-protective ability of LAVs needs to be further evaluated. ASFV has mutations, duplications, and loss of certain sequences in the genomes of different genotypes or even different strains of the same genotype, which may lead to changes in virulence [85][58].
3.2. Subunit Vaccines
Subunit vaccines are generally in the laboratory research stage, and there is still a huge gap between practical applications. It has been confirmed that some ASFV antigens have a certain protective effect [24,26][24][26]. Antibodies against p72 and p54 can block the adsorption of ASFV, and antibodies against p30 can block the internalization of the virus [87][59]. The key to subunit vaccines is to screen for those antigens or those epitopes of antigens that have definite protective effects [88][60]. In the presence of non-neutralizing antibodies, the virus-antibody complex formed is infectious. The virus bound by the non-neutralizing antibody is phagocytosed by macrophages, and the virus can still grow in the macrophage at this time, and the virus bound by the non-neutralizing antibody accelerates the replication of the virus [89,90][61][62].
Previous studies have noted a critical role for CD8+ T cells in protection, as demonstrated by using the virulent Portuguese ASFV isolate OUR/T88/1 [91][63]. DNA vaccines as well as vector vaccines have been attempted as alternative ASF vaccine platforms. In theory, DNA vaccines as well as vectors have better immunogenicity because the antigen can be expressed intracellularly and via MHC I, which is important for CD8+ T cell activation. Neither DNA vaccine, vector vaccine, nor prime-boost immunization strategies can provide complete protection [56,92,93,94][64][65][66][67]. Luckily, Goatley et al. cloned B602L, p72, p30, p54, E199L, EP153R, F317L, and MGF505-5R into adenovirus vectors for primary immunization, and into MVA vectors for booster immunization, then found the protective function up to 100% in pigs [30]. However, low levels of infectious virus remained in the immunized pigs until the end of the trial [30]. Future experiments should determine whether pigs can clear the infection and whether these vaccinated animals shed infectious virus [30].
Similar to screening for antigens for neutralizing antibodies, screening is also required to identify antigenic epitopes capable of inducing ASFV-specific T cells. Bosch-Camós used in silico prediction of ASFV protein CD8+ T cell epitopes and predicted a 19-mer peptide from MGF100-1L [76][68]. Netherton et al. predicted peptides corresponding to 133 proteins encoded by OUR T88/3, and screened 18 viral proteins that could be recognized by lymphocytes of ASF-immunized pigs [78][69]. The specific antigenic epitopes are determined by software analysis and verification in combination with relevant experiments, which guides the selection of antigens in the development of ASF vaccines.
References
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103.
- Mulumba-Mfumu, L.K.; Saegerman, C.; Dixon, L.K.; Madimba, K.C.; Kazadi, E.; Mukalakata, N.T.; Oura, C.A.L.; Chenais, E.; Masembe, C.; Stahl, K.; et al. African swine fever: Update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 2019, 66, 1462–1480.
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246.
- Sanchez, E.G.; Perez-Nunez, D.; Revilla, Y. Development of vaccines against African swine fever virus. Virus Res. 2019, 265, 150–155.
- Niemi, J.K. Impacts of African Swine Fever on Pigmeat Markets in Europe. Front Vet. Sci 2020, 7, 634.
- Mileto, P.; da Conceicao, F.; Stevens, V.; Cummins, D.; Certoma, A.; Neave, M.J.; Bendita da Costa Jong, J.; Williams, D.T. Complete Genome Sequence of African Swine Fever Virus Isolated from a Domestic Pig in Timor-Leste, 2019. Microbiol. Resour. Announc. 2021, 10, e0026321.
- Tran, H.T.T.; Truong, A.D.; Dang, A.K.; Ly, D.V.; Nguyen, C.T.; Chu, N.T.; Nguyen, H.T.; Dang, H.V. Genetic characterization of African swine fever viruses circulating in North Central region of Vietnam. Transbound. Emerg. Dis. 2021, 68, 1697–1699.
- Goonewardene, K.B.; Onyilagha, C.; Goolia, M.; Le, V.P.; Blome, S.; Ambagala, A. Superficial Inguinal Lymph Nodes for Screening Dead Pigs for African Swine Fever. Viruses 2022, 14, 83.
- African Swine Fever (ASF)—Situation Report 4. Available online: https://www.oie.int/app/uploads/2022/01/asf-situation-report-4.pdf (accessed on 18 January 2022).
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447.
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531.
- Liu, Y.; Zhang, X.; Qi, W.; Yang, Y.; Liu, Z.; An, T.; Wu, X.; Chen, J. Prevention and Control Strategies of African Swine Fever and Progress on Pig Farm Repopulation in China. Viruses 2021, 13, 2552.
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765.
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg. Microbes Infect. 2021, 10, 1–30.
- Borca, M.V.; Kutish, G.F.; Afonso, C.L.; Irusta, P.; Carrillo, C.; Brun, A.; Sussman, M.; Rock, D.L. An African swine fever virus gene with similarity to the T-lymphocyte surface antigen CD2 mediates hemadsorption. Virology 1994, 199, 463–468.
- Yáñez, R.J.; Rodríguez, J.M.; Nogal, M.L.; Yuste, L.; Enríquez, C.; Rodriguez, J.F.; Viñuela, E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 1995, 208, 249–278.
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634.
- Zhang, J.; Zhang, Y.; Chen, T.; Yang, J.; Yue, H.; Wang, L.; Zhou, X.; Qi, Y.; Han, X.; Ke, J.; et al. Deletion of the L7L-L11L Genes Attenuates ASFV and Induces Protection against Homologous Challenge. Viruses 2021, 13, 255.
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J. Virol. 2020, 94, e02017.
- Borca, M.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.; Gladue, D. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765.
- Hanh, T.; Phuong, L.; Huy, N.; Thuy, D.; Van Dung, N.; Gay, C.; Borca, M.; Gladue, D. African swine fever virus vaccine candidate ASFV-G-DI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis. 2021.
- Zhang, Y.; Ke, J.; Zhang, J.; Yang, J.; Yue, H.; Zhou, X.; Qi, Y.; Zhu, R.; Miao, F.; Li, Q.; et al. ASFV bearing an I226R gene-deletion elicits a robust immunity in pigs to African swine fever. J. Virol. 2021, 95, e0119921.
- Gladue, D.P.; Ramirez-Medina, E.; Vuono, E.; Silva, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Borca, M.V. Deletion of A137R gene from the pandemic strain of African swine fever virus is attenuated and offers protection against virulent pandemic virus. J. Virol. 2021, 95, e0113921.
- Gómez-Puertas, P.; Rodríguez, F.; Oviedo, J.; Brun, A.; Alonso, C.; Escribano, J. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 1998, 243, 461–471.
- Neilan, J.; Zsak, L.; Lu, Z.; Burrage, T.; Kutish, G.; Rock, D. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342.
- Ruiz-Gonzalvo, F.; Rodríguez, F.; Escribano, J. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 1996, 218, 285–289.
- Chen, X.; Yang, J.; Ji, Y.; Okoth, E.; Liu, B.; Li, X.; Yin, H.; Zhu, Q. Recombinant Newcastle disease virus expressing African swine fever virus protein 72 is safe and immunogenic in mice. Virol. Sin. 2016, 31, 150–159.
- Feng, Z.; Chen, J.; Liang, W.; Chen, W.; Li, Z.; Chen, Q.; Cai, S. The recombinant pseudorabies virus expressing African swine fever virus CD2v protein is safe and effective in mice. Virol. J. 2020, 17, 180.
- Murgia, M.V.; Mogler, M.; Certoma, A.; Green, D.; Monaghan, P.; Williams, D.T.; Rowland, R.R.R.; Gaudreault, N.N. Evaluation of an African swine fever (ASF) vaccine strategy incorporating priming with an alphavirus-expressed antigen followed by boosting with attenuated ASF virus. Arch. Virol. 2019, 164, 359–370.
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.S.; Montoya, M.; Sanchez-Cordon, P.J.; Taylor, G.; et al. A Pool of Eight Virally Vectored African Swine Fever Antigens Protect Pigs Against Fatal Disease. Vaccines 2020, 8, 234.
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582.
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066.
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274.
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Gallardo, C.; Pejsak, Z.; Belsham, G.J.; Rasmussen, T.B.; Botner, A. Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Vet. Microbiol. 2017, 211, 92–102.
- Pereira De Oliveira, R.; Hutet, E.; Duhayon, M.; Guionnet, J.M.; Paboeuf, F.; Vial, L.; Le Potier, M.F. Successful Infection of Domestic Pigs by Ingestion of the European Soft Tick, O. Erraticus That Fed on African Swine Fever Virus Infected Pig. Viruses 2020, 12, 300.
- Yang, B.; Shen, C.; Zhang, D.; Zhang, T.; Shi, X.; Yang, J.; Hao, Y.; Zhao, D.; Cui, H.; Yuan, X.; et al. Mechanism of interaction between virus and host is inferred from the changes of gene expression in macrophages infected with African swine fever virus CN/GS/2018 strain. Virol. J. 2021, 18, 170.
- De Oliveira, V.; Almeida, S.; Soares, H.; Crespo, A.; Marshall-Clarke, S.; Parkhouse, R. A novel TLR3 inhibitor encoded by African swine fever virus (ASFV). Arch. Virol. 2011, 156, 597–609.
- Garcia-Belmonte, R.; Perez-Nunez, D.; Pittau, M.; Richt, J.A.; Revilla, Y. African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway. J. Virol. 2019, 93, e02298-18.
- Correia, S.; Ventura, S.; Parkhouse, R.M. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 2013, 173, 87–100.
- Zhu, J.J.; Ramanathan, P.; Bishop, E.A.; O’Donnell, V.; Gladue, D.P.; Borca, M.V. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLoS ONE 2019, 14, e0223955.
- Liu, X.; Ao, D.; Jiang, S.; Xia, N.; Xu, Y.; Shao, Q.; Luo, J.; Wang, H.; Zheng, W.; Chen, N.; et al. African Swine Fever Virus A528R Inhibits TLR8 Mediated NF-kappaB Activity by Targeting p65 Activation and Nuclear Translocation. Viruses 2021, 13, 2046.
- Wang, X.; Wu, J.; Wu, Y.; Chen, H.; Zhang, S.; Li, J.; Xin, T.; Jia, H.; Hou, S.; Jiang, Y.; et al. Inhibition of cGAS-STING-TBK1 signaling pathway by DP96R of ASFV China 2018/1. Biochem. Biophys. Res. Commun. 2018, 506, 437–443.
- Zhuo, Y.; Guo, Z.; Ba, T.; Zhang, C.; He, L.; Zeng, C.; Dai, H. African Swine Fever Virus MGF360-12L Inhibits Type I Interferon Production by Blocking the Interaction of Importin alpha and NF-kappaB Signaling Pathway. Virol. Sin. 2020, 36, 176–186.
- Liu, H.; Zhu, Z.; Feng, T.; Ma, Z.; Xue, Q.; Wu, P.; Li, P.; Li, S.; Yang, F.; Cao, W.; et al. African swine fever virus E120R protein inhibits interferon-β production by interacting with IRF3 to block its activation. J. Virol. 2021, 95, e0082421.
- Yang, J.; Li, S.; Feng, T.; Zhang, X.; Yang, F.; Cao, W.; Chen, H.; Liu, H.; Zhang, K.; Zhu, Z.; et al. African Swine Fever Virus F317L Protein Inhibits NF-kappaB Activation To Evade Host Immune Response and Promote Viral Replication. mSphere 2021, 6, e0065821.
- Li, D.; Yang, W.; Li, L.; Li, P.; Ma, Z.; Zhang, J.; Qi, X.; Ren, J.; Ru, Y.; Niu, Q.; et al. African Swine Fever Virus MGF-505-7R Negatively Regulates cGAS-STING-Mediated Signaling Pathway. J. Immunol. 2021, 206, 1844–1857.
- Yang, K.; Huang, Q.; Wang, R.; Zeng, Y.; Cheng, M.; Xue, Y.; Shi, C.; Ye, L.; Yang, W.; Jiang, Y.; et al. African swine fever virus MGF505-11R inhibits type I interferon production by negatively regulating the cGAS-STING-mediated signaling pathway. Vet. Microbiol. 2021, 263, 109265.
- Li, D.; Zhang, J.; Yang, W.; Li, P.; Ru, Y.; Kang, W.; Li, L.; Ran, Y.; Zheng, H. African swine fever virus protein MGF-505-7R promotes virulence and pathogenesis by inhibiting JAK1- and JAK2-mediated signaling. J. Biol. Chem. 2021, 297, 101190.
- Huang, L.; Xu, W.; Liu, H.; Xue, M.; Liu, X.; Zhang, K.; Hu, L.; Li, J.; Liu, X.; Xiang, Z.; et al. African Swine Fever Virus pI215L Negatively Regulates cGAS-STING Signaling Pathway through Recruiting RNF138 to Inhibit K63-Linked Ubiquitination of TBK1. J. Immunol. 2021, 207, 2754–2769.
- Chaulagain, S.; Delhon, G.A.; Khatiwada, S.; Rock, D.L. African Swine Fever Virus CD2v Protein Induces beta-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells. Viruses 2021, 13, 1480.
- Teklue, T.; Sun, Y.; Abid, M.; Luo, Y.; Qiu, H.J. Current status and evolving approaches to African swine fever vaccine development. Transbound. Emerg. Dis. 2020, 67, 529–542.
- Monteagudo, P.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.; Vidal, E.; et al. BA71ΔCD2: A New Recombinant Live Attenuated African Swine Fever Virus with Cross-Protective Capabilities. J. Virol. 2017, 91, e01058-17.
- Ramirez-Medina, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Pina-Pedrero, S.; Zhu, J.; Rodriguez, F.; Borca, M.V.; et al. Deletion of E184L, a putative DIVA target from the pandemic strain of African swine fever virus, produces a reduction in virulence and protection against virulent challenge. J. Virol. 2021, 96, e01419-21.
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The progressive adaptation of a georgian isolate of African swine fever virus to vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J. Virol. 2015, 89, 2324–2332.
- Wang, T.; Wang, L.; Han, Y.; Pan, L.; Yang, J.; Sun, M.; Zhou, P.; Sun, Y.; Bi, Y.; Qiu, H.J. Adaptation of African swine fever virus to HEK293T cells. Transbound. Emerg. Dis. 2021, 68, 2853–2866.
- Borca, M.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D. A Cell Culture-Adapted Vaccine Virus against the Current African Swine Fever Virus Pandemic Strain. J. Virol. 2021, 95, e0012321.
- Takenouchi, T.; Kitani, H.; Suzuki, S.; Nakai, M.; Fuchimoto, D.I.; Tsukimoto, M.; Shinkai, H.; Sato, M.; Uenishi, H. Immortalization and Characterization of Porcine Macrophages That Had Been Transduced with Lentiviral Vectors Encoding the SV40 Large T Antigen and Porcine Telomerase Reverse Transcriptase. Front. Vet. Sci. 2017, 4, 132.
- Bao, J.; Wang, Q.; Lin, P.; Liu, C.; Li, L.; Wu, X.; Chi, T.; Xu, T.; Ge, S.; Liu, Y.; et al. Genome comparison of African swine fever virus China/2018/AnhuiXCGQ strain and related European p72 Genotype II strains. Transbound. Emerg. Dis. 2019, 66, 1167–1176.
- Gomez-Puertas, P.; Rodriguez, F.; Oviedo, J.M.; Ramiro-Ibanez, F.; Ruiz-Gonzalvo, F.; Alonso, C.; Escribano, J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996, 70, 5689–5694.
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.A.J.; et al. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35.
- Argilaguet, J.M.; Perez-Martin, E.; Gallardo, C.; Salguero, F.J.; Borrego, B.; Lacasta, A.; Accensi, F.; Diaz, I.; Nofrarias, M.; Pujols, J.; et al. Enhancing DNA immunization by targeting ASFV antigens to SLA-II bearing cells. Vaccine 2011, 29, 5379–5385.
- Lokhandwala, S.; Petrovan, V.; Popescu, L.; Sangewar, N.; Elijah, C.; Stoian, A.; Olcha, M.; Ennen, L.; Bray, J.; Bishop, R.P.; et al. Adenovirus-vectored African Swine Fever Virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Vet. Microbiol. 2019, 235, 10–20.
- Oura, C.; Denyer, M.; Takamatsu, H.; Parkhouse, R. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 86, 2445–2450.
- Chen, T.; Chen, Q.; Ge, L.; Liu, Z.; Zhou, X.; Qi, Y.; Miao, F.; Wu, T.; Wang, L.; Yang, J.; et al. Characterizing Lansibai-2 pigs, a special breed in China, resistant to African swine fever. Chin. J. Vet. Sci. 2020, 40, 665–672.
- Sunwoo, S.Y.; Perez-Nunez, D.; Morozov, I.; Sanchez, E.G.; Gaudreault, N.N.; Trujillo, J.D.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-Protein Vaccination Strategy Does Not Protect from Challenge with African Swine Fever Virus Armenia 2007 Strain. Vaccines 2019, 7, 12.
- Lacasta, A.; Ballester, M.; Monteagudo, P.L.; Rodriguez, J.M.; Salas, M.L.; Accensi, F.; Pina-Pedrero, S.; Bensaid, A.; Argilaguet, J.; Lopez-Soria, S.; et al. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J. Virol. 2014, 88, 13322–13332.
- Lopera-Madrid, J.; Osorio, J.E.; He, Y.; Xiang, Z.; Adams, L.G.; Laughlin, R.C.; Mwangi, W.; Subramanya, S.; Neilan, J.; Brake, D.; et al. Safety and immunogenicity of mammalian cell derived and Modified Vaccinia Ankara vectored African swine fever subunit antigens in swine. Vet. Immunol. Immunop. 2017, 185, 20–33.
- Bosch-Camos, L.; Lopez, E.; Collado, J.; Navas, M.J.; Blanco-Fuertes, M.; Pina-Pedrero, S.; Accensi, F.; Salas, M.L.; Mundt, E.; Nikolin, V.; et al. M448R and MGF505-7R: Two African Swine Fever Virus Antigens Commonly Recognized by ASFV-Specific T-Cells and with Protective Potential. Vaccines 2021, 9, 508.
- Jancovich, J.K.; Chapman, D.; Hansen, D.T.; Robida, M.D.; Loskutov, A.; Craciunescu, F.; Borovkov, A.; Kibler, K.; Goatley, L.; King, K.; et al. Immunization of Pigs by DNA Prime and Recombinant Vaccinia Virus Boost To Identify and Rank African Swine Fever Virus Immunogenic and Protective Proteins. J. Virol. 2018, 92, e02219-17.
More
