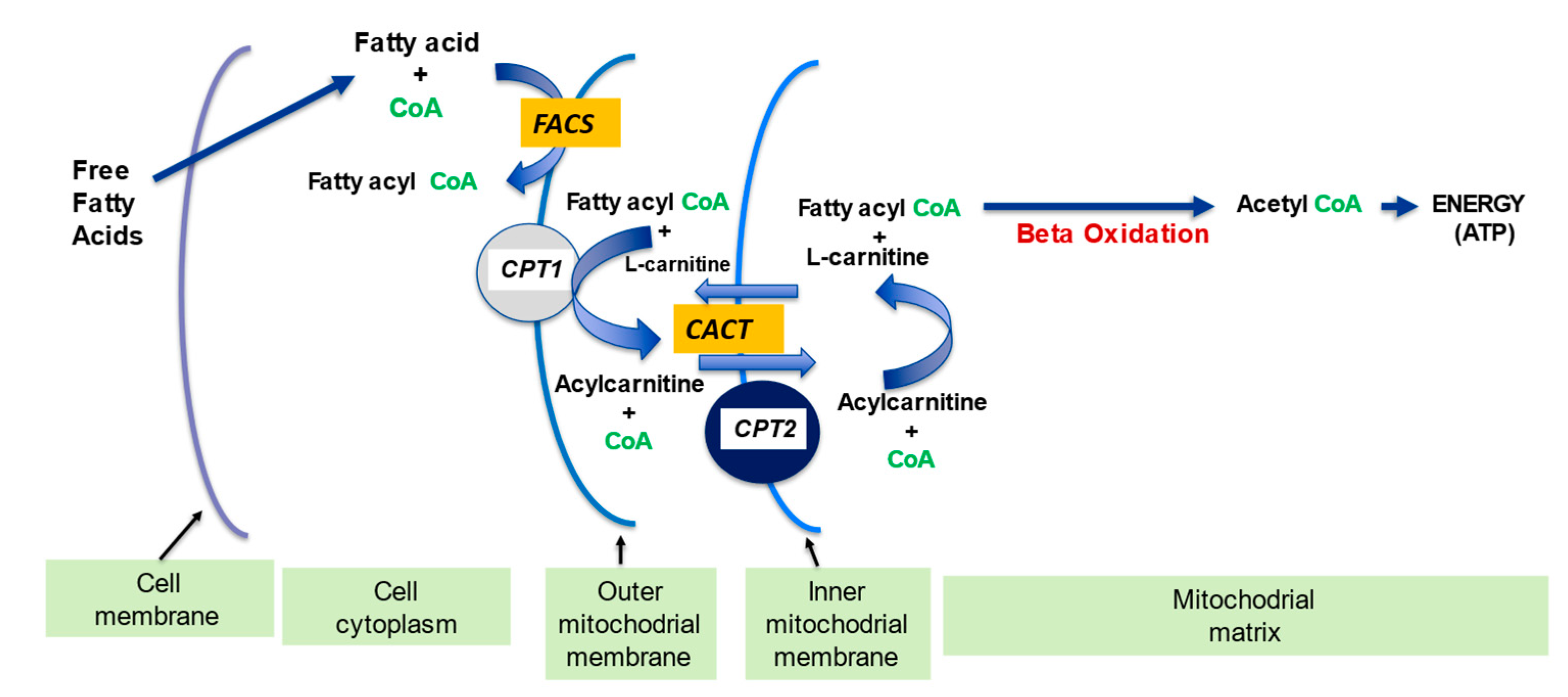
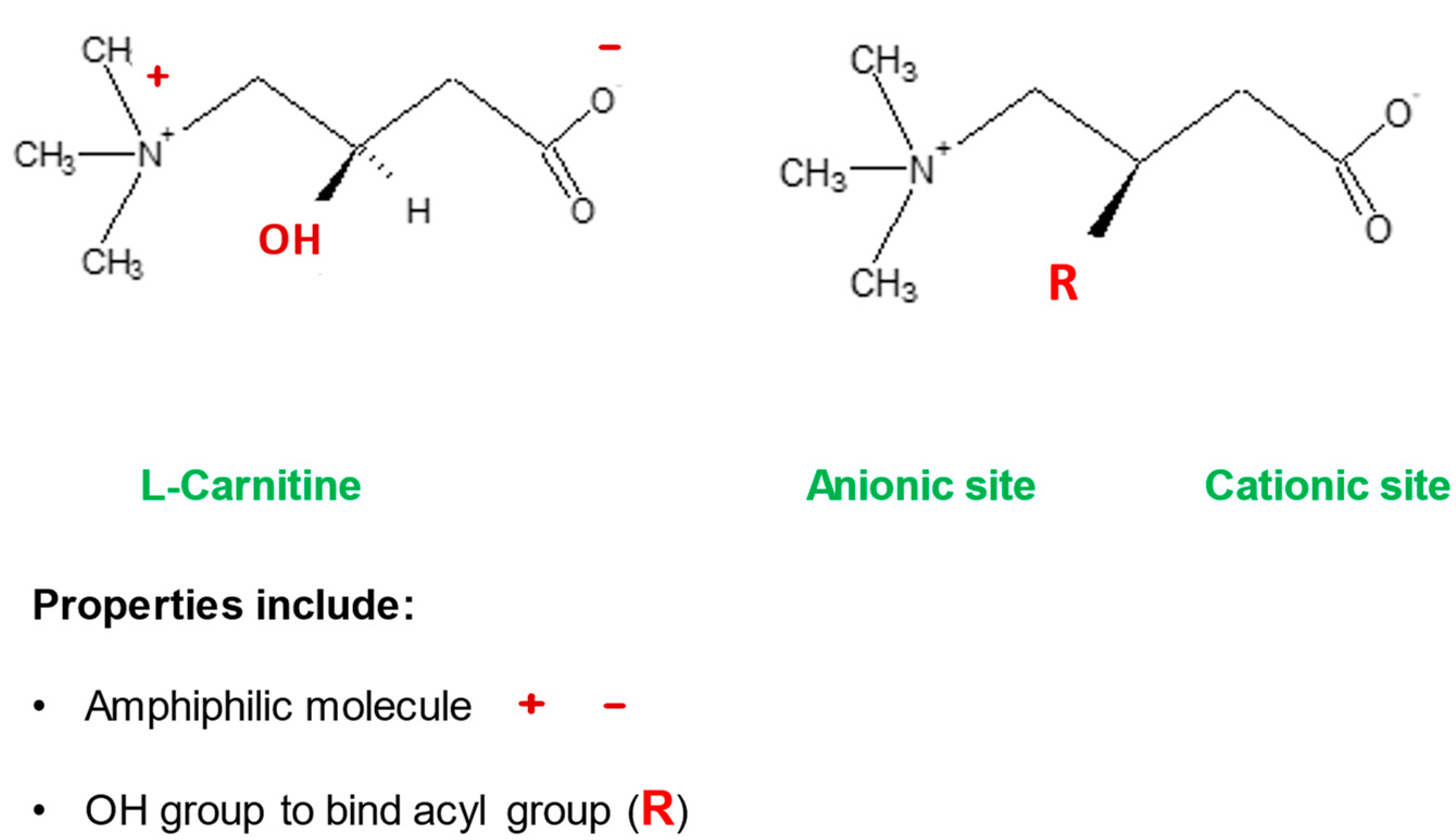
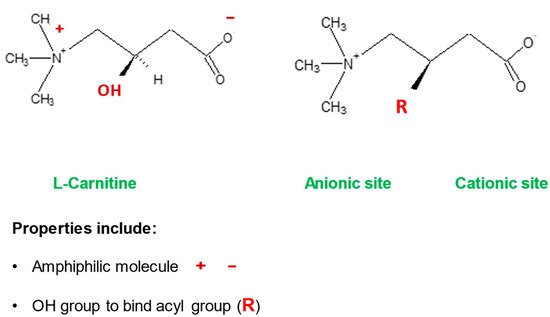
Mitochondria control cellular fate by various mechanisms and are key drivers of cellular metabolism. Although the main function of mitochondria is energy production, they are also involved in cellular detoxification, cellular stabilization, as well as control of ketogenesis and glucogenesis. Conditions like neurodegenerative disease, insulin resistance, endocrine imbalances, liver and kidney disease are intimately linked to metabolic disorders or inflexibility and to mitochondrial dysfunction. Mitochondrial dysfunction due to a relative lack of micronutrients and substrates is implicated in the development of many chronic diseases. l-carnitine is a vital molecule that is found in all living cells. It is a quaternary amine (3-hydroxy-4-N-trimethylaminobutyrate) whose main function in mammalian cells is the transfer of long chain fatty acids across the inner mitochondrial membrane for β- oxidation and generation of ATP energy.
- mitochondrial function
- l-carnitine
- fatty acid oxidation
- glycolysis
- ketogenesis
- beta oxidation
1. Introduction
2. l-Carnitine, Mitochondria and Cellular Metabolism
| l | -Carnitine (LC) Function | Description | References |
|---|---|---|---|
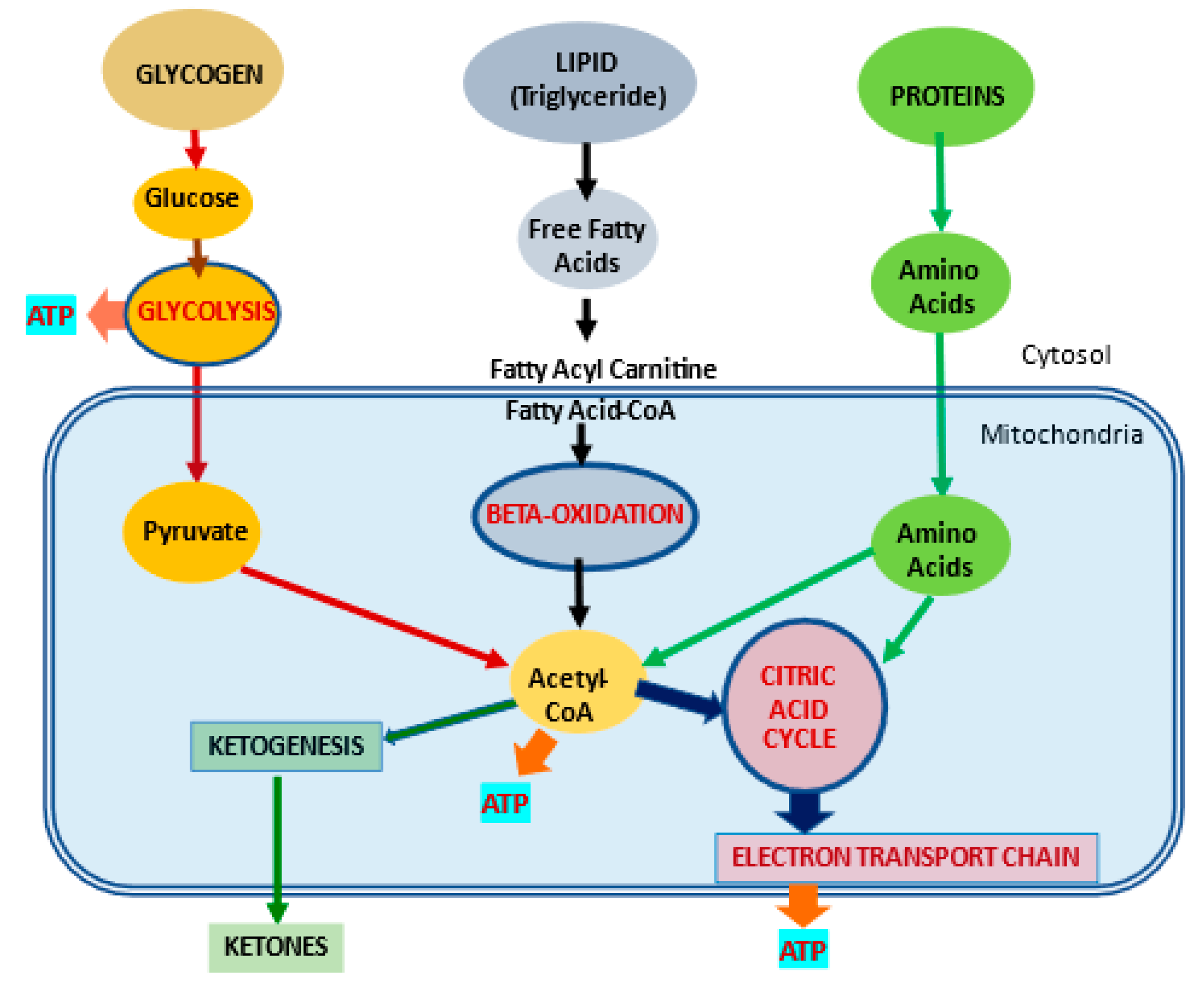
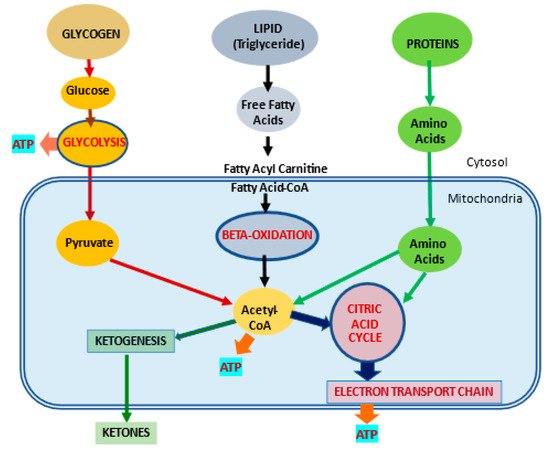
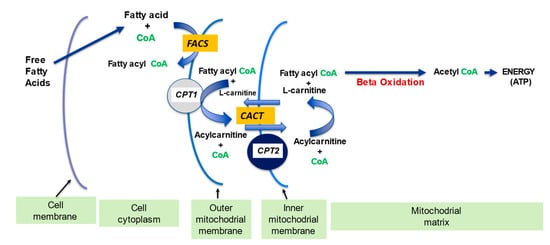
| Fatty acid metabolism | LC transports LCFA across inner mitochondrial membrane for subsequent beta oxidation and ATP production | [4][5] | |
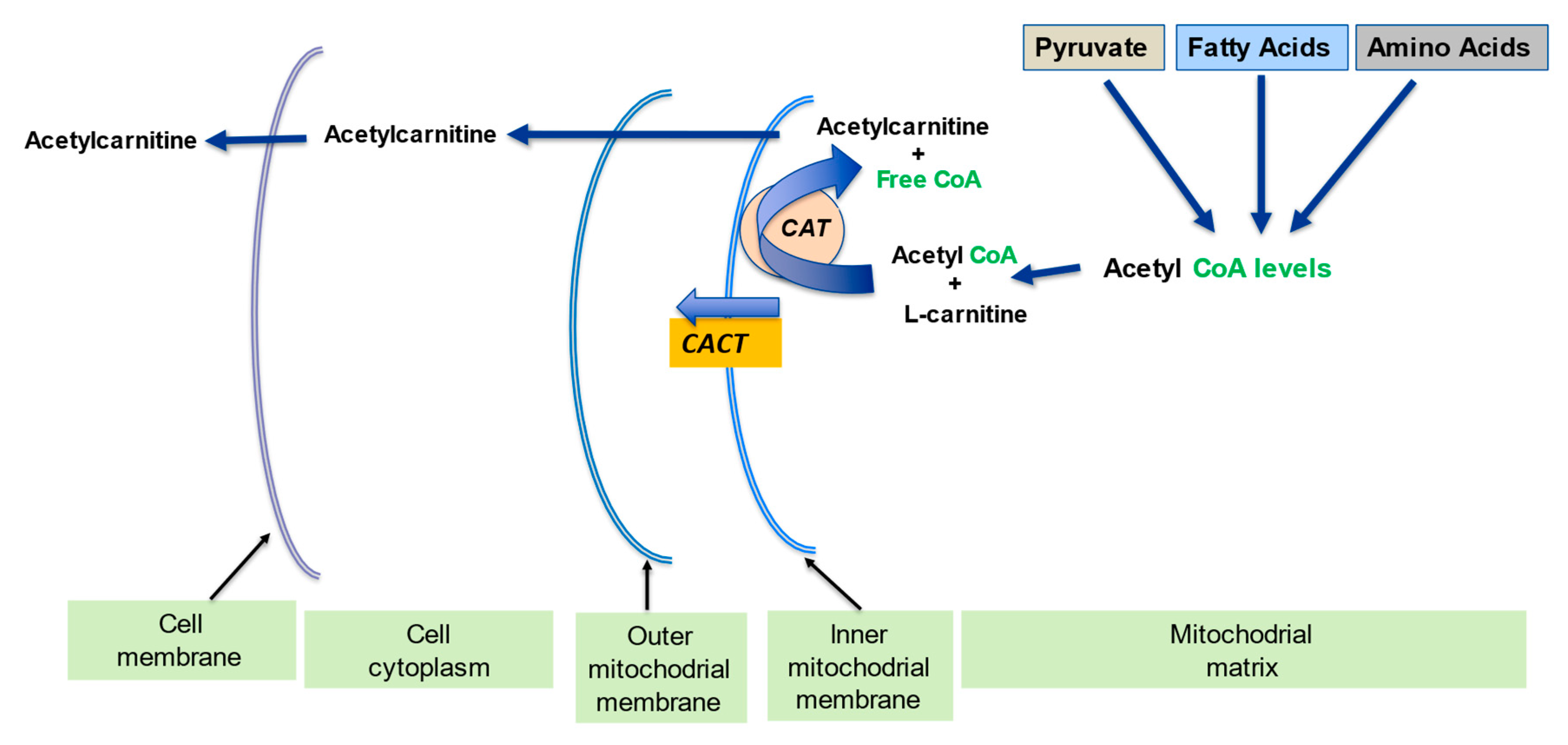
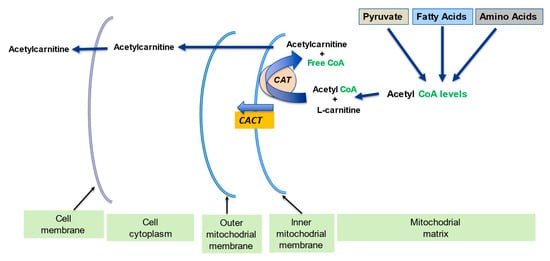
| Regulation of mitochondrial acetyl-CoA/CoA ratio and acyl-CoA/CoA ratio | LC forms an effective transport system for acetyl or acyl groups out of the mitochondria to maintain free CoA levels important for glycolysis and other processes | [1][5] | |
| Detoxification of potentially toxic metabolite | LC binds acyl residues and helps in their elimination thereby maintaining metabolic flexibility | [7] | |
| Stabilization of cell membranes | LC helps stabilize cell membranes via its effects on acetylation of membrane phospholipids and surface membrane effects | [8][9] | |
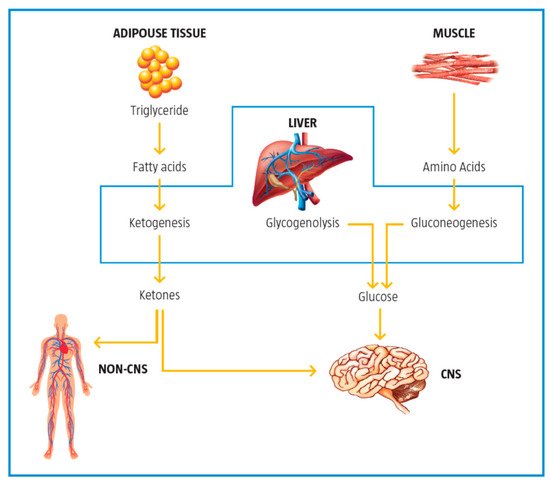
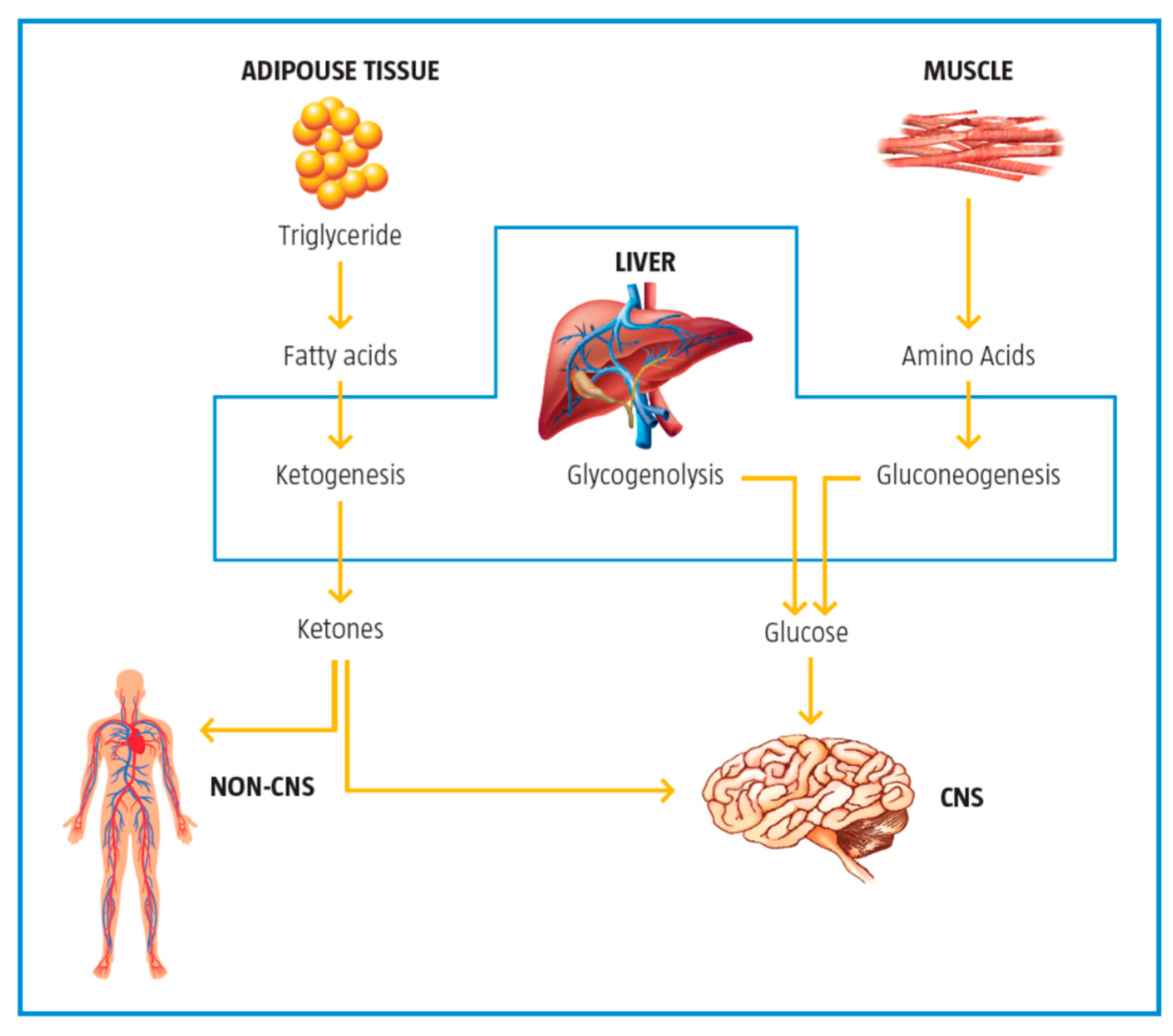
| Control of ketogenesis and gluconeogenesis | LC affects ketogenesis by transporting free fatty acids into mitochondria for subsequent use in the production of ketones in the mitochondria. | [10] |
2.1. l-Carnitine and Fatty Acid Metabolism
2.2. Regulation of the Mitochondrial Acetyl-CoA/CoA Ratio and Acyl-CoA/CoA Ratio
2.3. Detoxification of Toxic Metabolites
2.4. Stabilization of Cell Membranes
2.5. Control of Ketogenesis and Gluconeogenesis
3. Metabolic Inflexibility
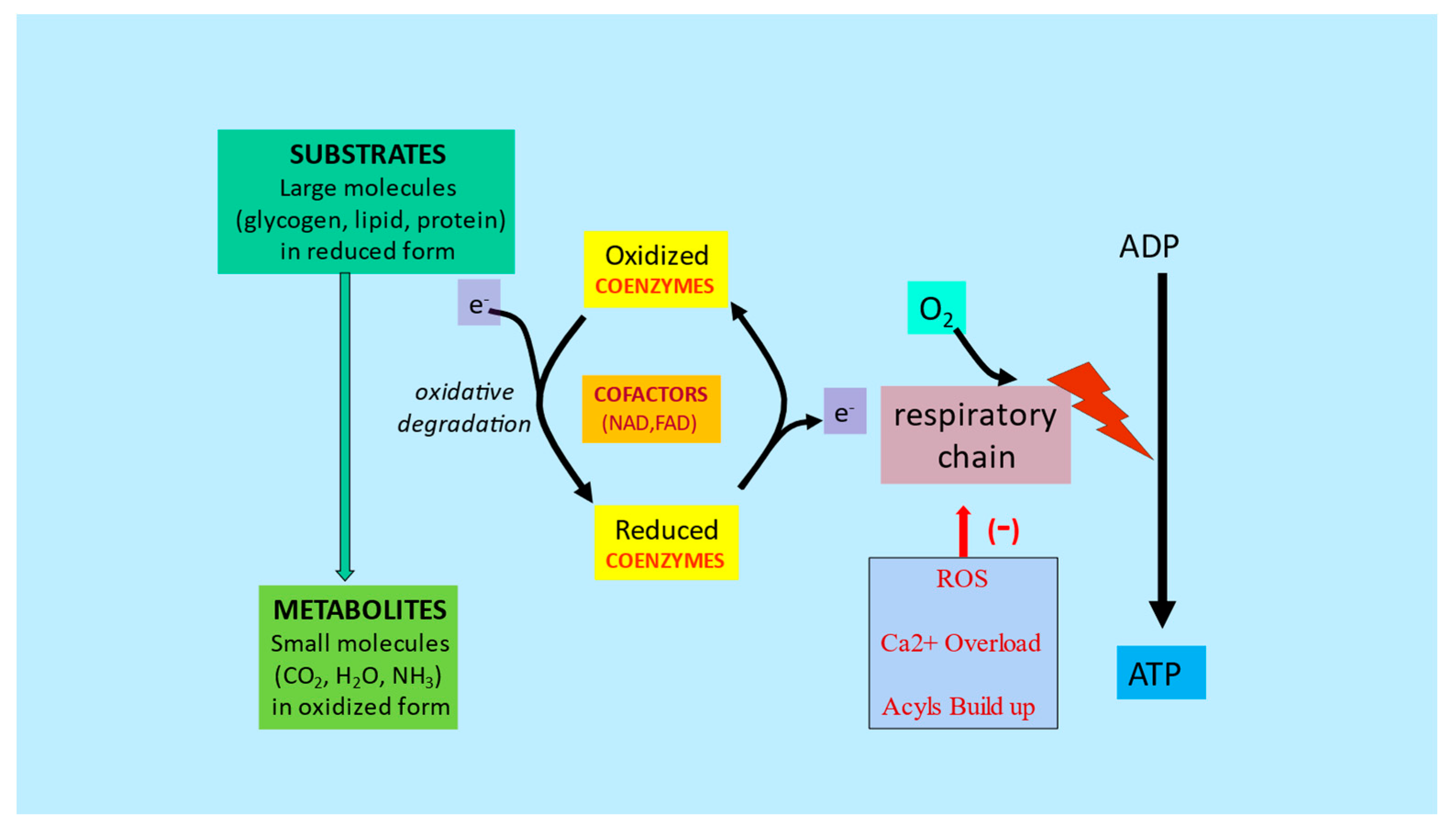
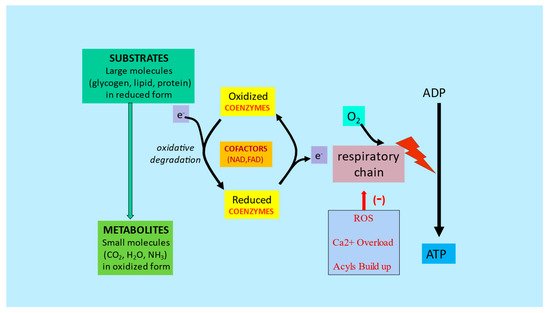
References
- Virmani, A.; Pinto, L.; Bauermann, O.; Zerelli, S.; Diedenhofen, A.; Binienda, Z.K.; Ali, S.F.; van der Leij, F.R. The carnitine palmitoyl transferase (CPT) system and possible relevance for neuropsychiatric and neurological conditions. Mol. Neurobiol. 2015, 52, 826–836.
- Mynatt, R.L. Carnitine and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, S45–S49.
- Xu, Y.; Jiang, W.; Chen, G. l-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 333–338.
- Wang, Z.Y.; Liu, Y.Y.; Liu, G.H.; Lu, H.B.; Mao, C.Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97.
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219.
- Wang, D.D.; Mao, Y.Z.; He, S.M.; Yang, Y.; Chen, X. Quantitative efficacy of l-carnitine supplementation on glycemic control in type 2 diabetes mellitus patients. Expert Rev. Clin. Pharmacol. 2021, 14, 919–926.
- Carter, A.L.; Abney, T.O.; Lapp, D.F. Biosynthesis and metabolism of carnitine. J. Child Neurol. 1995, 10 (Suppl. 2), S3–S7.
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 15, 77–85.
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283.
- Wanders, R.J.A.; Visser, G.; Ferdinandusse, S.; Vaz, F.M.; Houtkooper, R.H. Mitochondrial fatty acid oxidation disorders: Laboratory diagnosis, pathogenesis, and the complicated route to treatment. J. Lipid Atheroscler. 2020, 9, 313–333.
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233.
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Biophys. Acta 2000, 1486, 1–17.
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 1, 417–429.
- Fingerhut, R.; Ensenauer, R.; Röschinger, W.; Arnecke, R.; Olgemöller, B.; Roscher, A.A. Stability of acylcarnitines and free carnitine in dried blood samples: Implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal. Chem. 2009, 1, 3571–3575.
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435.
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44.
- Drosatos, K.; Schulze, P.C. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 2013, 10, 109–121.
- Ferreira, G.C.; McKenna, M.C. l-carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675.
- Hülsmann, W.C.; Dubelaar, M.L.; Lamers, J.M.; Maccari, F. Protection by acyl-carnitines and phenylmethylsulfonyl fluoride of rat heart subjected to ischemia and reperfusion. Biochim. Biophys. Acta 1985, 30, 62–66.
- Virmani, A.; Binienda, Z. Role of carnitine esters in brain neuropathology. Mol. Aspects Med. 2004, 25, 533–549.
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16.
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493179/ (accessed on 28 January 2022).
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine inborn errors of metabolism. Molecules 2019, 24, 3251.
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262.
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036.
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517.
- Virmani, A.; Pinto, L.; Binienda, Z.; Ali, S. Food, nutrigenomics, and neurodegeneration—Neuroprotection by what you eat! Mol. Neurobiol. 2013, 48, 353–362.
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485.
- Berezhnov, A.V.; Fedotova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting cellular mechanisms of long-chain acylcarnitines-driven cardiotoxicity: Disturbance of calcium homeostasis, activation of Ca2+-dependent phospholipases, and mitochondrial energetics collapse. Int. J. Mol. Sci. 2020, 10, 7461.
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970.
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216.
- Haigh, J.L.; New, L.E.; Filippi, B.M. Mitochondrial Dynamics in the Brain Are Associated with Feeding, Glucose Homeostasis, and Whole-Body Metabolism. Front. Endocrinol. 2020, 11, 580879.
- van de Weijer, T.; Sparks, L.M.; Phielix, E. Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS ONE 2013, 8, e51648.
- Stark, R.; Reichenbach, A.; Andrews, Z.B. Hypothalamic carnitine metabolism integrates nutrient and hormonal feedback to regulate energy homeostasis. Mol. Cell. Endocrinol. 2015, 418 Pt 1, 9–16.
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995.
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 32–45.