Gymnema inodorum (GI) is an indigenous medicinal plant and functional food in Thailand that has recently helped to reduce plasma glucose levels in healthy humans. It is renowned for the medicinal properties of gymnemic acid and its ability to suppress glucose absorption. However, the effects of gymnemic acids on adipogenesis that contribute to the accumulation of adipose tissues associated with obesity remain unknown. The present study aimed to determine the effects of gymnemic acids derived from GI tea on adipogenesis. We purified and identified GiA-7 and stephanosides C and B from GI tea that inhibited adipocyte differentiation in 3T3-L1 cells. These compounds also suppressed the expression of peroxisome proliferator-activated receptor gamma (Pparγ)-dependent genes, indicating that they inhibit lipid accumulation and the early stage of 3T3-L1 preadipocyte differentiation. Only GiA-7 induced the expression of uncoupling protein 1 (Ucp1) and pparγ coactivator 1 alpha (Pgc1α), suggesting that GiA-7 induces mitochondrial activity and beige-like adipocytes. This is the first finding of stephanosides C and B in
(GI) is an indigenous medicinal plant and functional food in Thailand
that has recently helped to reduce plasma glucose levels in healthy humans. It is renowned for the
medicinal properties of gymnemic acid and its ability to suppress glucose absorption. However,
the effects of gymnemic acids on adipogenesis that contribute to the accumulation of adipose
tissues associated with obesity remain unknown. The present study aimed to determine the
effects of gymnemic acids derived from GI tea on adipogenesis. We purified and identified GiA-7
and stephanosides C and B from GI tea that inhibited adipocyte differentiation in 3T3-L1 cells.
These compounds also suppressed the expression of peroxisome proliferator-activated receptor gamma
(Pparγ)-dependent genes, indicating that they inhibit lipid accumulation and the early stage of 3T3-L1
preadipocyte differentiation. Only GiA-7 induced the expression of uncoupling protein 1 (Ucp1) and
pparγ coactivator 1 alpha (Pgc1α), suggesting that GiA-7 induces mitochondrial activity and beige-like
adipocytes. This is the first finding of stephanosides C and B in
Gymnema inodorum. Our results suggested that GiA-7 and stephanosides C and B from GI tea could help to prevent obesity.
. Our results
suggested that GiA-7 and stephanosides C and B from GI tea could help to prevent obesity.
- Gymnema inodorum
- adipogenesis
- gymnemic acid
- obesity
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition:Gymnema inodorum (GI) is an indigenous medicinal plant and functional food in Thailand that has recently helped to reduce plasma glucose levels in healthy humans. It is renowned for the medicinal properties of gymnemic acid and its ability to suppress glucose absorption.
1. Introduction
Gymnema sylvestre is a species of the genus Gymnema that is popular in India for reducing glucose levels, suppressing glucose absorption and preventing type 2 diabetes [1][2][3][4][5].
Gymnema inodorum (GI) is a species of same genus that is indigenous to Thailand, particularly in the northern region, where it is widely consumed. The effects of GI on glucose absorption and blood glucose levels have recently been investigated [6][7][8]. We previously found that extracts of GI leaves decreased blood glucose in alloxan-induced diabetic rats [9] that comprise a popular model with which to study type 1 diabetes mellitus. Alloxan selectively destroys insulin production in beta cells, which consequently results in high blood glucose levels [10]. However, about 90% of patients with diabetes have type 2 diabetes mellitus (DM) which is induced by a lack of exercise and inappropriate eating habits [11]. However, obesity is the leading risk factor for type 2 DM, and it also greatly increases the risk of fatty liver disease, atherosclerosis, metabolic diseases, insulin resistance and hypertension [12][13]. Obesity is characterized at the cellular level as being differentiated from preadipocytes. White adipose tissue (WAT) is specialized to store excess energy as triglycerides composed of fatty acids. Inhibiting preadipocyte differentiation can prevent the initiation and progression of obesity [14][15].
The differentiation of 3T3-L1 fibroblast-like cells into adipocyte-like cells stimulated by insulin and synthetic glucocorticoids is a popular model of adipogenesis and lipid metabolism in vitro [16][17]. Therefore, we applied the inhibition of 3T3-L1 cell differentiation to screen gymnemic acid extracted from GI tea. Gymnemic acid is an oleanane-type triterpene glycoside [16][17] that can exist as a single entity or as a mixture of several related compounds [18][19]. The major saponin fraction in
Gymnema sylvestre
peroxisome proliferator-activated receptor gamma (Ppar
), CCAAT/enhancer-binding protein alpha (Cebp
), cluster of differentiation 36 (Cd36), fatty acid synthase (Fasn), ppar
coactivator 1 alpha (Pgc1α), lipin-1, adipose triglyceride lipase (Atgl), hormone-sensitive lipase (Hsl), sterol regulatory element-binding protein (Srebp)-1c, uncoupling protein 1 (Ucp1), glucose transporter type 4 (Glut4)
fatty acid binding protein 4 (Fabp4)
2. Results and Discussion
We measured the ability of the crude 10–100% methanol and ethanol fractions of gymnemic acid to inhibit 3T3-L1 cell differentiation. We found that the 90% methanol fraction was the most powerful inhibitor (). We then found that components 2, 3, 5 and 6 among the six components separated by HPLC from this fraction () significantly inhibited 3T3-L1 cell differentiation (). Component 5 was the most powerful inhibitor. The inhibition of 3T3-L1 cell differentiation by component 5 was concentration-dependent. Component 6 also strongly inhibited 3T3-L1 cell differentiation. However, the yield of HPLC fraction 3 was very low. Therefore, HPLC fractions No. 2, 5 and 6 were further purified, and their structures were identified by NMR and mass spectrometry.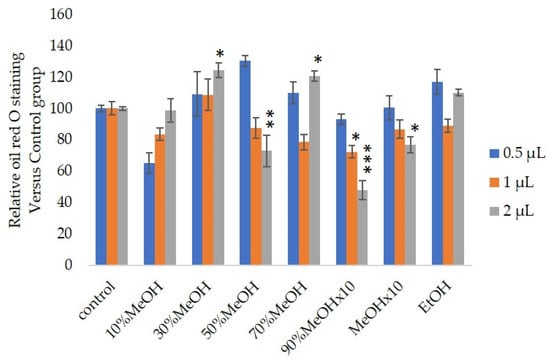
Figure 1.
n
p
p
p
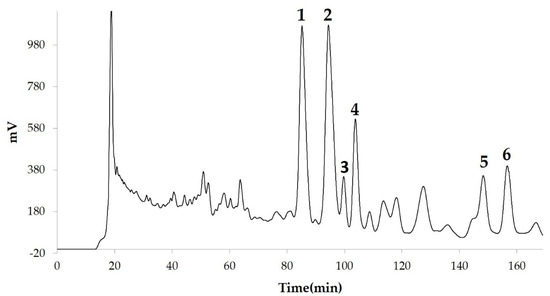
Figure 2.
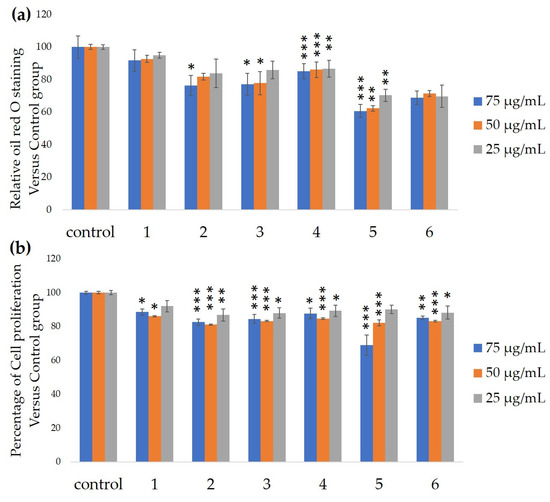
Figure 3.
a
b
n
p
p
p
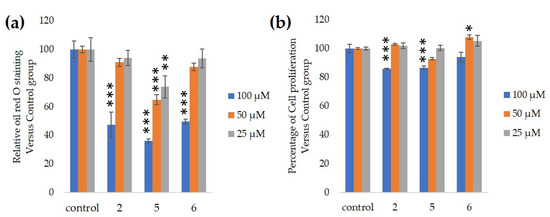
Figure 4.
a
b
n
p
p
p
Hsl
Atgl
Pparγ
Hsl
Atgl gene expressions in adipocytes in vitro [21][22]. Our results suggested that these compounds downregulated
Hsl
Atgl
Pparγ gene expression. Lipin-1 functions in lipid droplet biogenesis during adipocyte differentiation and generates diacylglycerol for lipid synthesis [23]. Lipin-1 is important for the process of TG accumulation during the early stage of adipogenesis. Lipin-1 is a key factor for adipocyte maturation and maintenance by regulating
Pparγ
Cebpα [24].
Lipin-1
Pparγ
lipin-1
Pparγ
Cebpα
lipin-1
Pgc1α
Srebp-1c upregulation [27][28]. The present study found that only GiA-7 induced
Srebp-1c
Pgc1α. Srebp-1c is also a key regulator of adipocytes and is involved in lipid metabolism [29][30]. These findings suggest that GiA-7 regulates mitochondrial biogenesis through the Srebp-1c-dependent upregulation of Pgc1α. GiA-7 also inhibits lipid accumulation in 3T3-L1 preadipocytes by downregulating adipogenic transcription factors and genes associated with lipid accumulation. Both
Pgc1α
Ucp1
Pgc1α
Ucp1
Ucp1
Ucp1. These findings suggest that GiA-7 inhibits the differentiation of white adipocytes and, also, induces beige-like adipocytes in 3T3-L1 mouse preadipocytes. Comprehensive profiles of gene expressions indicate that the characteristics of human brown and mouse beige adipocytes are compatible [29][30]. The activation of human brown adipocytes was recently examined as a possible novel therapeutic treatment for obesity [31]. Thus, GiA-7 might serve as a novel treatment for obesity in humans by inducing brown adipocytes.
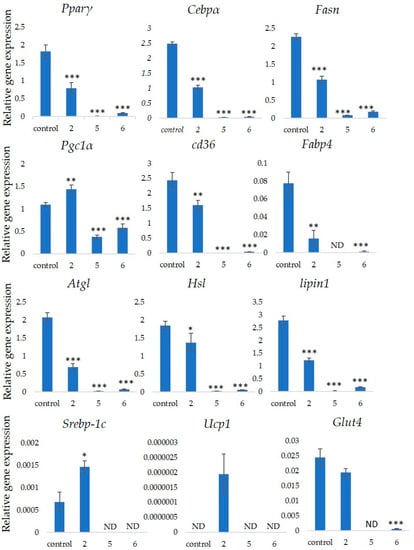
Figure 5.
Gymnema inodorum
n
p
p
p
3. Discussions and Conclusions
Gymnema inodorum
Lipin-1, Pparγ, Cebp
, Fasn, Cd36
Fabp4
Ucp1
Pgc1α
