Photodynamic therapy (PDT) is emerging as a significant complementary or alternative approach for cancer treatment. PDT drugs act as photosensitisers, which upon using appropriate wavelength light and in the presence of molecular oxygen, can lead to cell death. Herein, we reviewed the general characteristics of the different generation of photosensitisers and outlined the emergence of rhenium (Re) and more specifically, Re(I) tricarbonyl complexes ias a new generation of metal-based photosensitisers for PDTphotodynamic therapy that are of great interest in multidisciplinary research. The photophysical properties and structures of Re(I) complexes discussed in this review are summarised to determine basic features and similarities among the structures that are important for their phototoxic activity and future investigations. We also discussed Re(I) complexes in conjunction with the advancement of two-photon PDT, drug combination study, nanomedicine, and photothermal therapy to overcome the limitation of such complexes, which generally absorb short wavelengths.
- cancer
- photodynamic therapy
- rhenium(I) tricarbonyl complexes
- photosensitisers
1. Introduction
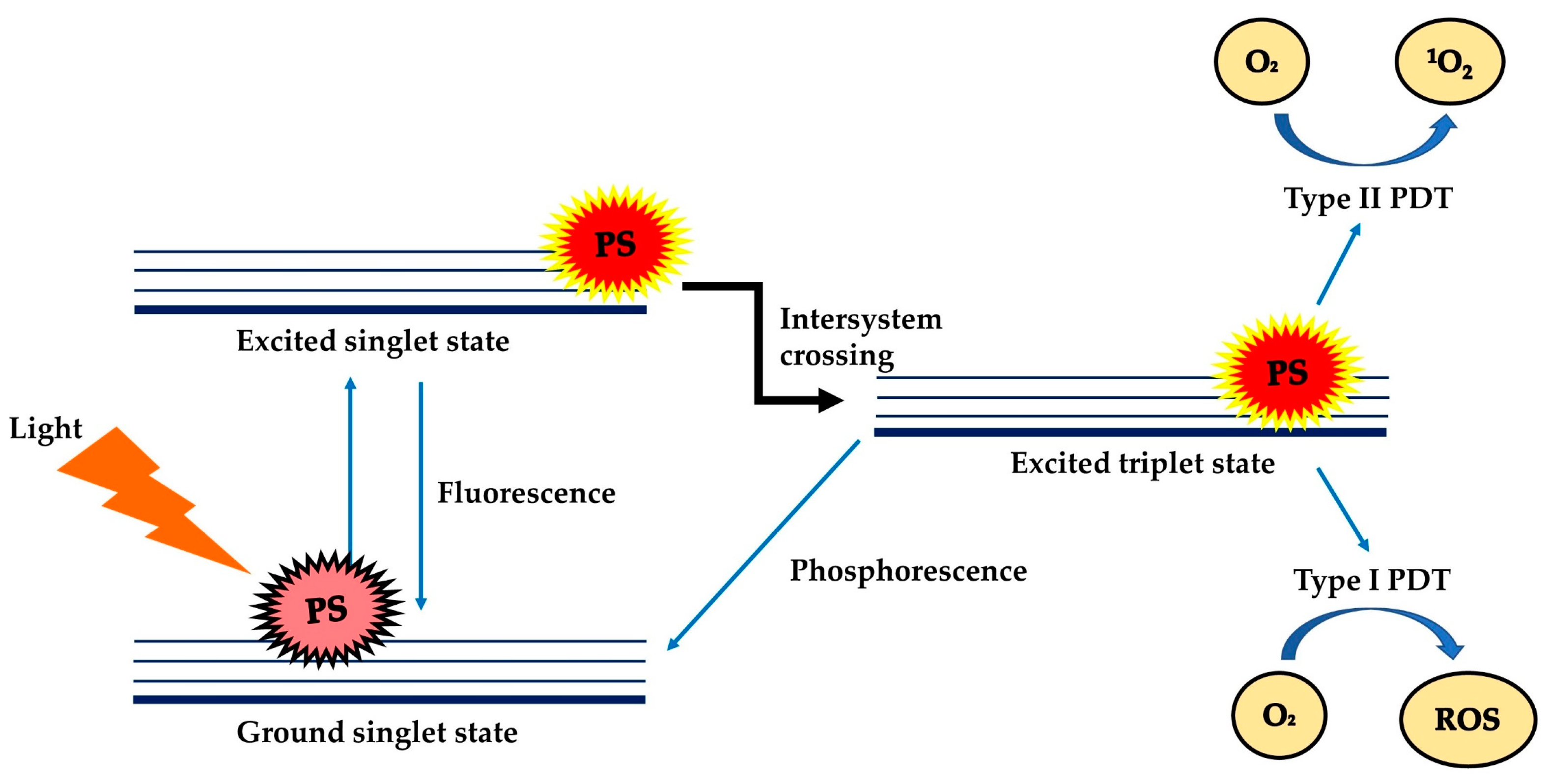
Photodynamic Therapy (PDT) is a treatment modality that uses harmless visible light to activate non-toxic light-excitable molecules (i.e., the photosensitisers), for the generation of cytotoxic reactive oxygen species (ROS, type I photoactivation) and singlet oxygen (type II photoactivation) from molecular oxygen (
)
within or at near vicinity to cancer cells, to cause cell death and tissue necrosis
[8]
. PDT was claimed to be relatively selective and safe compared to classical anticancer therapies due to the use of harmless therapeutic agents during the process, liberty of activating only the photosensitisers (PSs) at the tumour region via selective exposure of the tumour to light, and increased tendency of the targeted photosensitisers to accumulate at the tumour site
. PDT can be used repetitively without causing resistance to tumour or hypersensitivity to normal tissue, as a single agent or in conjunction with other anticancer therapies such as chemotherapy and radiotherapy
.
Figure 1.
Schematic illustration of the mechanism of PDT which involves Type I and Type II mechanisms. PS: photosensitiser; O
2
: oxygen;
1
O
2
: singlet oxygen; ROS: reactive oxygen species.
At present, a number of PSs have received approval from the FDA for the treatment of cancer (e.g., skin, pancreatic and other cancers—refer to
)
[8]
and non-cancer disorders (e.g., verteporfin for age-related macular degeneration treatment and 5-aminolevulinic acid for moderate to severe acne vulgaris)
[15]
. Despite the advantages, the extensive clinical use of PDT was limited by the inherent weaknesses of common photosensitisers, such as water insolubility, aggregation tendency in the physiological environment, and requirement of a rich oxygen environment for the production of singlet oxygen/ROS
. In recent years, Re(I) complexes have been increasingly explored as a new alternative choice of photosensitisers for PDT
. This review gives a brief account of the development of anticancer PDT and assesses the potential of Re(I) complexes as potent photosensitisers for PDT.
Table 1.
Application of clinically approved photosensitisers for treatment of cancer.
| Photosensitisers | Application | References |
|---|
| NPe6 (Talaporfin sodium) | Non-small cell lung carcinoma | [21] |
| Motexafin lutetium | Prostate cancer | [22] |
| Temoporfin | Head, neck, prostate and pancreatic cancers | [23][24] |
| Porfimer sodium | Obstructive oesophageal, lung, bladder and cervical cancers | [25][26] |
| 2-(1-Hexyloxyethyl)-2-devinyl pyropheophorbide-a | Head, lung and neck cancers, basal cell carcinoma | [27][28] |
| Hexaminolevulinate | Bladder cancer | [29] |
| Methyl aminolevulinate | Basal cell carcinoma | [30] |
| Aluminium phthalocyanine tetrasulfonate | Lung, breast, skin and stomach cancers | [31] |
| Padeliporfin | Early-stage of prostate cancer | [32] |
| Verteporfin | Basal cell carcinoma | [33][34] |
2. Potential of Re(I) tricarbonyl complex as photosensitisers
Studies done by several research groups including Gasser’s group, Megger’s group, Alex’s group and others have proved that Re(I) tricarbonyl complexes are potential new generation of photosensitisers which may generate free radicals to kill the cancer cells at higher wavelength by tuning of the functional group or ligands attach to it. Structural modifications have been done on the published Re(I) tricarbonyl complexes to improve the photo-absorption profiles and phototoxic activity towards HeLa cells and PC-3 cells. Significant result (difference of 10-20 µM) before and after modification of the structures can be seen in the published data. For example, Gasser et al. further explored the use of a photolabile protecting group (PLPG) to enable the release of Re(I) tricarbonyl complex at low irradiation energy (1.2 J cm
−2
). Such strategy in combining targeting with peptides and irradiation-dependent release of the PLPG-Re complexes further increased the phototoxicity of these compounds against cancer cells (HeLa and PC-3, IC
50
: 9.3 µM) while, at the same time, reduced the phototoxic effects towards non-cancerous cells (MRC-5, IC
50
: 20.5 µM)
.
Re(CO)
3
complexes is an attractive candidate for further investigation in the effort to discover new and effective photosensitisers for PDT. The introduction of more ring structures or fluorine or methyl groups as displayed by reported complexes will be the structural strategy to improve lipophilicity and phototoxic effect of new Re(I) tricarbonyl complexes. Moreover, Re(I) complexes can be included in formulations with various nanoparticles such as liposomes, liquid crystalline nanoparticles or micelles to enhance its solubility and delivery to the cancer cells. This class of compounds can also be paired with synergistic agents like current chemotherapeutic agents to improve cellular targeting effect and treatment outcomes. Advancement in combination therapies discovery may unlock a new platform to strengthen the antitumor effects and enhance clinical outcomes. From our review, only a few Re(I) complexes advanced into the
in vivo
or clinical phase
. The available
in vivo
studies mainly focused on Re(I) tricarbonyl complexes tested on the cytotoxic activity but not on PDT. This might be due to the short UV wavelength required for excitation. With the emergence of two-photon laser technology, Re(I) complex can be further investigated to result in a deeper tissue penetration through photoactivation with two times higher wavelength. Besides, the Re(I) complex may also be studied further in photothermal therapy to improve its anticancer activity through heat energy. As Re(I) complexes have been most frequently studied in cell culture, more preclinical and clinical studies are expected to be carried out in the future.
3. Conclusions
The development of Re(I) tricarbonyl complexes has unlocked a new avenue for the treatment of cancer via photodynamic therapy to improve the overall survival rate of cancer patients. To achieve this, researchers and clinicians have to improvise their understandings of tumour immunology, novel complexes, and optimise the timing of photodynamic therapy. We believed that these novel complexes represent a promising alternative in all types of cancer, thus progressing from bench to bedside at a swift pace. Re(I) complexes as photosensitisers in photodynamic therapy will bring a bright future ahead for patients suffering from this aggressive and challenging killer.
References
- Ramaswamy Bhuvaneswari; Yik Yuen Gan; Khee Chee Soo; Malini Olivo; The effect of photodynamic therapy on tumor angiogenesis. Cellular and Molecular Life Sciences 2009, 66, 2275-2283, 10.1007/s00018-009-0016-4.
- Nancy L. Oleinick; Helen H. Evans; The photobiology of photodynamic therapy: cellular targets and mechanisms.. Radiation Research 1998, 150, S146, 10.2307/3579816.
- Ana P. Castano; Pawel Mroz; Michael R. Hamblin; Photodynamic therapy and anti-tumour immunity. Nature Cancer 2006, 6, 535-545, 10.1038/nrc1894.
- Raymond Bonnett; Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chemical Society Reviews 1995, 24, 19, 10.1039/cs9952400019.
- Anna Leonidova; Vanessa Pierroz; Riccardo Rubbiani; Yanjun Lan; Anita G. Schmitz; Andres Kaech; Roland K. O. Sigel; Stefano Ferrari; Gilles Gasser; Photo-induced uncaging of a specific Re(i) organometallic complex in living cells. Chemical Science 2014, 5, 4044-4056, 10.1039/c3sc53550a.
- M.-F. Zuluaga; N. Lange; Combination of photodynamic therapy with anti-cancer agents.. Current Medicinal Chemistry 2008, 15, 1655-1673, 10.2174/092986708784872401.
- Robertson, C.A.; Evans, D.H.; Abrahamse, H.; Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. . J. Photochem. Photobiol. B Biol. 2009, 96, 1-8.
- Stephen G. Bown; A Z Rogowska; D E Whitelaw; W R Lees; L B Lovat; P Ripley; L Jones; P Wyld; A Gillams; A W R Hatfield; et al. Photodynamic therapy for cancer of the pancreas. Gut 2002, 50, 549-557.
- Macrene Alexiades-Armenakas; Laser-mediated photodynamic therapy. Clinics in Dermatology 2006, 24, 16-25, 10.1016/j.clindermatol.2005.10.027.
- Brian C. Wilson; M. S. Patterson; Lothar Lilge; Implicit and explicit dosimetry in photodynamic therapy: a New paradigm. Lasers in Medical Science 1997, 12, 182-199, 10.1007/bf02765099.
- Dominic J. Robinson; Henriëtte S. Bruijn; Nynke Veen; Stanley B. Brown; Mark R. Stringer; Willem M. Star; Fluorescence Photobleaching of ALA-induced Protoporphyrin IX during Photodynamic Therapy of Normal Hairless Mouse Skin: The Effect of Light Dose and Irradiance and the Resulting Biological Effect. Photochemistry and Photobiology 1998, 67, 140-149, 10.1562/0031-8655(1998)067<0140:fpoaip>2.3.co;2.
- I. A. Boere; Dominic J. Robinson; H S De Bruijn; J Van Den Boogert; H W Tilanus; H J Sterenborg; R W De Bruin; Monitoring in situ dosimetry and protoporphyrin IX fluorescence photobleaching in the normal rat esophagus during 5-aminolevulinic acid photodynamic therapy.. Photochemistry and Photobiology 2003, 78, 271.
- Roger Ackroyd; C Kelty; N Brown; M Reed; The history of photodetection and photodynamic therapy.. Photochemistry and Photobiology 2001, 74, 656.
- Allison, R.R.; Sibata, C.H.; Oncologic photodynamic therapy photosensitizers: A clinical review. . Photodiagnosis Photodyn. Ther. 2010, 7, 61-75.
- Lihteh Wu; Robert P. Murphy; Photodynamic therapy: a new approach to the treatment of choroidal neovascularization secondary to age-related macular degeneration. Current Opinion in Ophthalmology 1999, 10, 217-220, 10.1097/00055735-199906000-00011.
- Brown, J.M.; Tumor hypoxia in cancer therapy. . Methods Enzymol. 2007, 435, 295-321.
- Agata Nowak-Stępniowska; Paulina Pergoł; Alfreda Padzik-Graczyk; [Photodynamic method of cancer diagnosis and therapy--mechanisms and applications].. Postępy Biochemii 2013, 59, 53-63.
- Zivile Luksiene; Photodynamic therapy: mechanism of action and ways to improve the efficiency of treatment.. Medicina 2003, 39, 1137-1150.
- Anna Leonidova; Vanessa Pierroz; Riccardo Rubbiani; Jakob Heier; Stefano Ferrari; Gilles Gasser; Towards cancer cell-specific phototoxic organometallic rhenium(i) complexes. Dalton Trans. 2014, 43, 4287-4294, 10.1039/c3dt51817e.
- Igor Kitanovic; Suzan Can; Hamed Alborzinia; Ana Kitanovic; Vanessa Pierroz; Anna Leonidova; Antonio Pinto; Priv. Doz. Dr. Bernhard Spingler; Priv. Doz. Dr. Stefano Ferrari; Roberto Molteni; et al.Andreas SteffenNils Metzler-NolteDr. Stefan WölflGilles Gasser A Deadly Organometallic Luminescent Probe: Anticancer Activity of a ReIBisquinoline Complex. Chemistry - A European Journal 2014, 20, 2496-2507, 10.1002/chem.201304012.
- Masakazu Kimura; Kuniharu Miyajima; Masakazu Kojika; Takafumi Kono; Harubumi Kato; Photodynamic Therapy (PDT) with Chemotherapy for Advanced Lung Cancer with Airway Stenosis. International Journal of Molecular Sciences 2015, 16, 25466-25475, 10.3390/ijms161025466.
- K.L. Du Md; R. Mick; T.M. Busch; T.C. Zhu; J.C. Finlay; G. Yu; A.G. Yodh; S.B. Malkowicz; D. Smith; R. Whittington; et al.D. StrippS.M. Hahn Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers in Surgery and Medicine 2006, 38, 427-434, 10.1002/lsm.20341.
- Triesscheijn, M.; Ruevekamp, M.; Aalders, M.; Baas, P.; Stewart, F.A.; Outcome of mTHPC mediated photodynamic therapy is primarily determined by the vascular response. Photochem. Photobiol. 2005, 81, 1161-1167.
- Stanley B Brown; Elizabeth A Brown; Ian Walker; The present and future role of photodynamic therapy in cancer treatment. The Lancet Oncology 2004, 5, 497-508, 10.1016/s1470-2045(04)01529-3.
- John D. Breskey; Steven E. Lacey; Benjamin J. Vesper; William A. Paradise; James A. Radosevich; Michael D. Colvard; Photodynamic Therapy: Occupational Hazards and Preventative Recommendations for Clinical Administration by Healthcare Providers. Photomedicine and Laser Surgery 2013, 31, 398-407, 10.1089/pho.2013.3496.
- Tom Reynolds; Photodynamic Therapy Expands Its Horizons. JNCI: Journal of the National Cancer Institute 1997, 89, 112-114, 10.1093/jnci/89.2.112.
- David A. Bellnier; William R. Greco; Hector Nava; Gregory M. Loewen; Allan R. Oseroff; Thomas J. Dougherty; Mild skin photosensitivity in cancer patients following injection of Photochlor (2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a; HPPH) for photodynamic therapy. Cancer Chemotherapy and Pharmacology 2005, 57, 40-45, 10.1007/s00280-005-0015-6.
- Tarak D. Mody; Pharmaceutical development and medical applications of porphyrin-type macrocycles. Journal of Porphyrins and Phthalocyanines 2000, 4, 362-367, 10.1002/(sici)1099-1409(200006/07)4:4<362::aid-jpp250>3.0.co;2-z.
- Peter Hillemanns; Karl-Ulrich Petry; Philipp Soergel; Pierre Collinet; Katty Ardaens; Julia Gallwas; Alexander Luyten; Christian Dannecker; Efficacy and safety of hexaminolevulinate photodynamic therapy in patients with low-grade cervical intraepithelial neoplasia. Lasers in Surgery and Medicine 2014, 46, 456-461, 10.1002/lsm.22255.
- Aisling E. O’Connor; William M. Gallagher; Annette Byrne; Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochemistry and Photobiology 2009, 85, 1053-1074, 10.1111/j.1751-1097.2009.00585.x.
- Arnold, R.; Dyes and pigments: New research. Nova Science: Hauppauge, 2009, NY, USA.
- Idan Ashur; Ruth Goldschmidt; Iddo Pinkas; Yoram Salomon; Grzegorz Szewczyk; Tadeusz Sarna; Avigdor Scherz; Photocatalytic Generation of Oxygen Radicals by the Water-Soluble Bacteriochlorophyll Derivative WST11, Noncovalently Bound to Serum Albumin. The Journal of Physical Chemistry A 2009, 113, 8027-8037, 10.1021/jp900580e.
- Michel Sickenberg; Ursula Schmidt-Erfurth; Joan W. Miller; Constantin J. Pournaras; Leonidas Zografos; Bertrand Piguet; Guy Donati; Horst Laqua; Irene Barbazetto; Evangelos S. Gragoudas; et al.Anne-Marie LaneReginald BirngruberHubert Van Den BerghH. Andrew StrongUlrike ManjurisTodd GrayMario FsadniNeil M. Bressler A Preliminary Study of Photodynamic Therapy Using Verteporfin for Choroidal Neovascularization in Pathologic Myopia, Ocular Histoplasmosis Syndrome, Angioid Streaks, and Idiopathic Causes. Archives of Ophthalmology 2000, 118, 327-336, 10.1001/archopht.118.3.327.
- Kirste J Mellish; Stanley B. Brown; Verteporfin: a milestone in opthalmology and photodynamic therapy. Expert Opinion on Pharmacotherapy 2001, 2, 351-361, 10.1517/14656566.2.2.351.
- Anja Kastl; Sandra Dieckmann; Kathrin Wähler; Timo Völker; Lena Kastl; Anna Lena Merkel; Adina Vultur; Batool Shannan; Klaus Harms; Dr. Matthias Ocker; et al.Dr. Wolfgang J. ParakMeenhard HerlynDr. Eric Meggers Rhenium Complexes with Visible-Light-Induced Anticancer Activity. ChemMedChem 2013, 8, 924-927, 10.1002/cmdc.201300060.
- Alex Man-Hei Yip; Justin Shum; Hua-Wei Liu; Huipeng Zhou; Meiqi Jia; Niu Niu; Yongxin Li; Cong Yu; Kenneth Kam-Wing Lo; Luminescent Rhenium(I)–Polypyridine Complexes Appended with a Perylene Diimide or Benzoperylene Monoimide Moiety: Photophysics, Intracellular Sensing, and Photocytotoxic Activity. Chemistry – A European Journal 2019, 25, 8970-8974, 10.1002/chem.201900345.
- Knopf, K.M.; Murphy, B.L.; Macmillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J.; In vitro anticancer activity and in vivo biodistribution of rhenium(I) tricarbonyl aqua complexes.. J. Am. Chem. Soc. 2017, 139, 14303-14314.
- Collery, P.; Mohsen, A.; Kermagoret, A.; Corre, S.; Bastian, G.; Tomas, A.; Wei, M.; Santoni, F.; Guerra, N.; Desmaële, D.; et al.et al. Antitumor activity of a rhenium(I)-diselenoether complex in experimental models of human breast cancer. . Investig. New Drugs 2015, 33, 848-860.
- Collery, P.; Santoni, F.; Ciccolini, J.; Tran, T.N.N.; Mohsen, A.; Desmaele, D.; Dose effect of rhenium(I)-diselenoether as anticancer drug in resistant breast tumor-bearing mice after repeated administrations. Anticancer Res. 2016, 36, 6051-6057.
- Collery, P.; Santoni, F.; Ciccolini, J.; Tran, T.N.N.; Mohsen, A.; Desmaele, D.; Dose effect of rhenium(I)-diselenoether as anticancer drug in resistant breast tumor-bearing mice after repeated administrations. Anticancer Res. 2016, 36, 6051-6057.