Anthocyanins are water-soluble, colored compounds of the flavonoid class, abundantly found in the fruits, leaves, roots, and other parts of the plants. The fruit berries are prime sources and exhibit different colors. The anthocyanins utility as traditional medicament for liver protection and cure, and importance as strongest plants-based anti-oxidants have conferred these plants products different biological activities. These activities include anti-inflammation, liver protective, analgesic, and anti-cancers, which have provided the anthocyanins an immense commercial value, and has impelled their chemistry, biological activity, isolation, and quality investigations as prime focus.
- anthocyanins
- traditional medicine
- liver protection
- hepatocellular longevity
- hepatic carcinoma
- anti-oxidant
- anti-inflammation
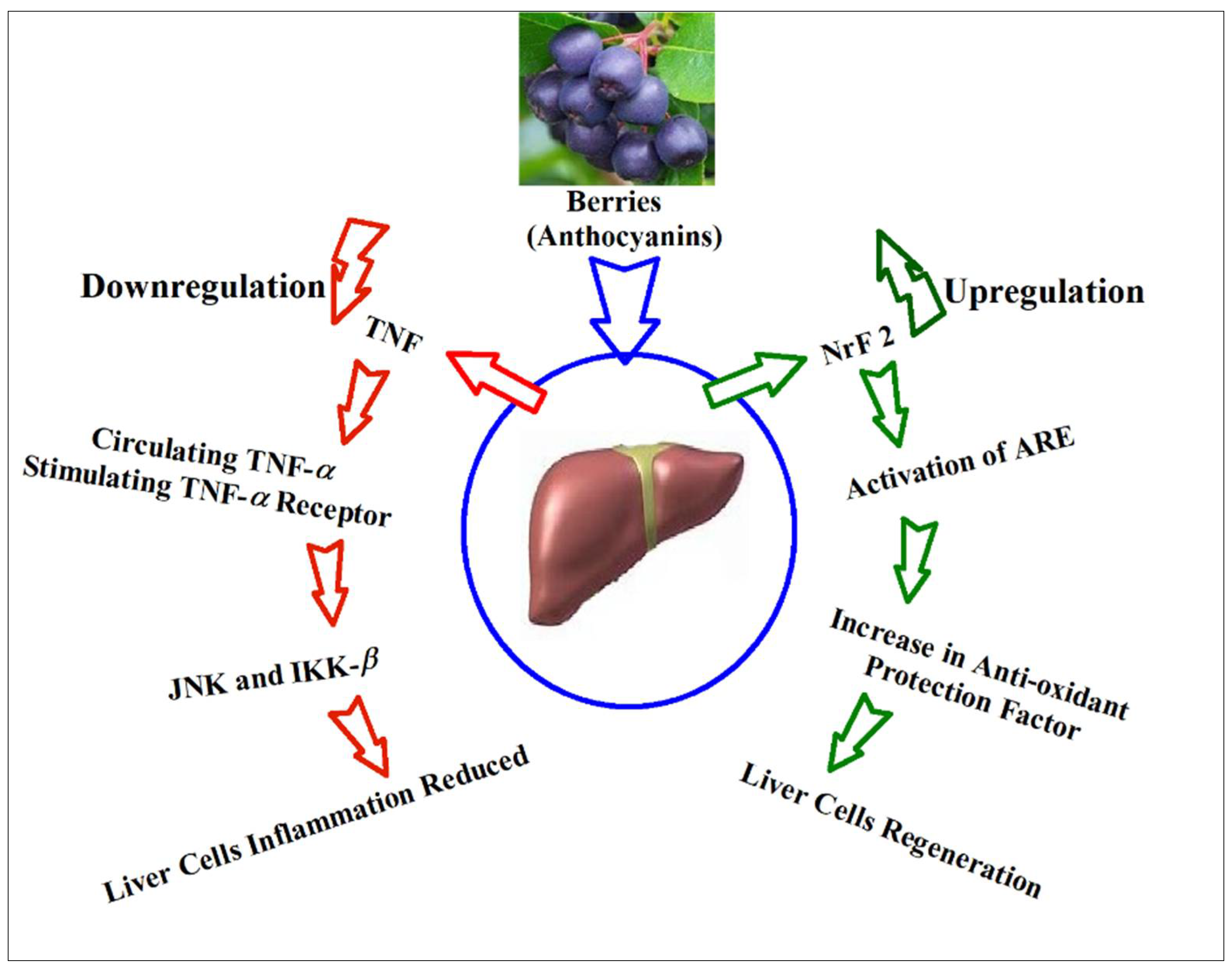
- NrF2
- TNF-α
1. Introduction
2. Anthocyanins’ Aesthetics, and Plant Kingdom’s Distribution
3. Anthocyanins Roles in the Plants
4. Chemistry of the Anthocyanins, Structures, and the Structural Variants
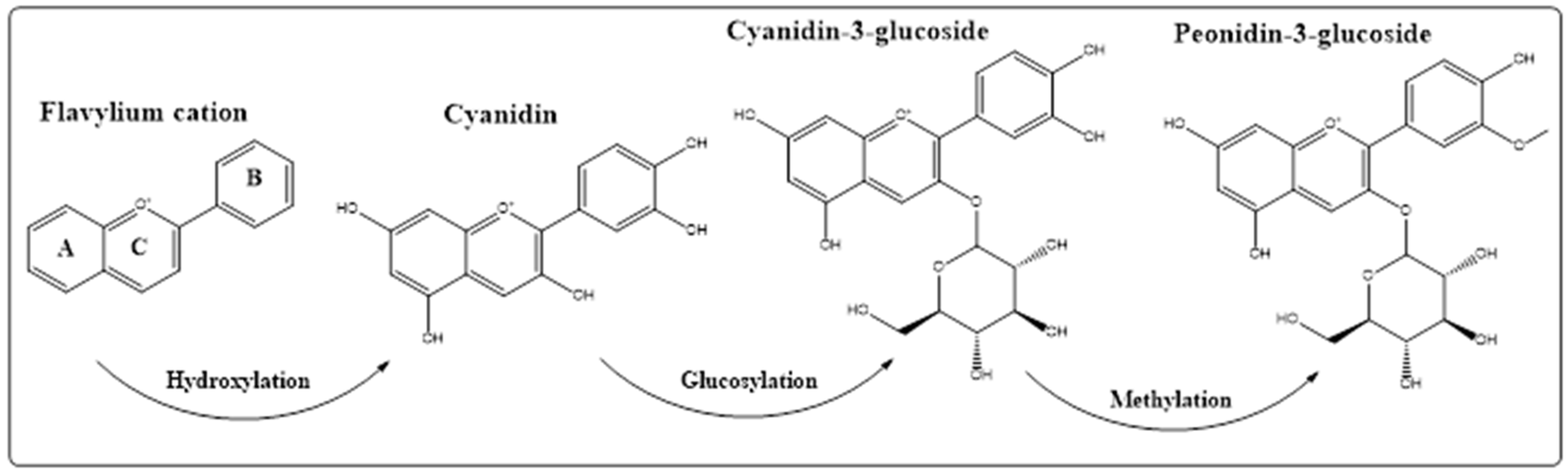
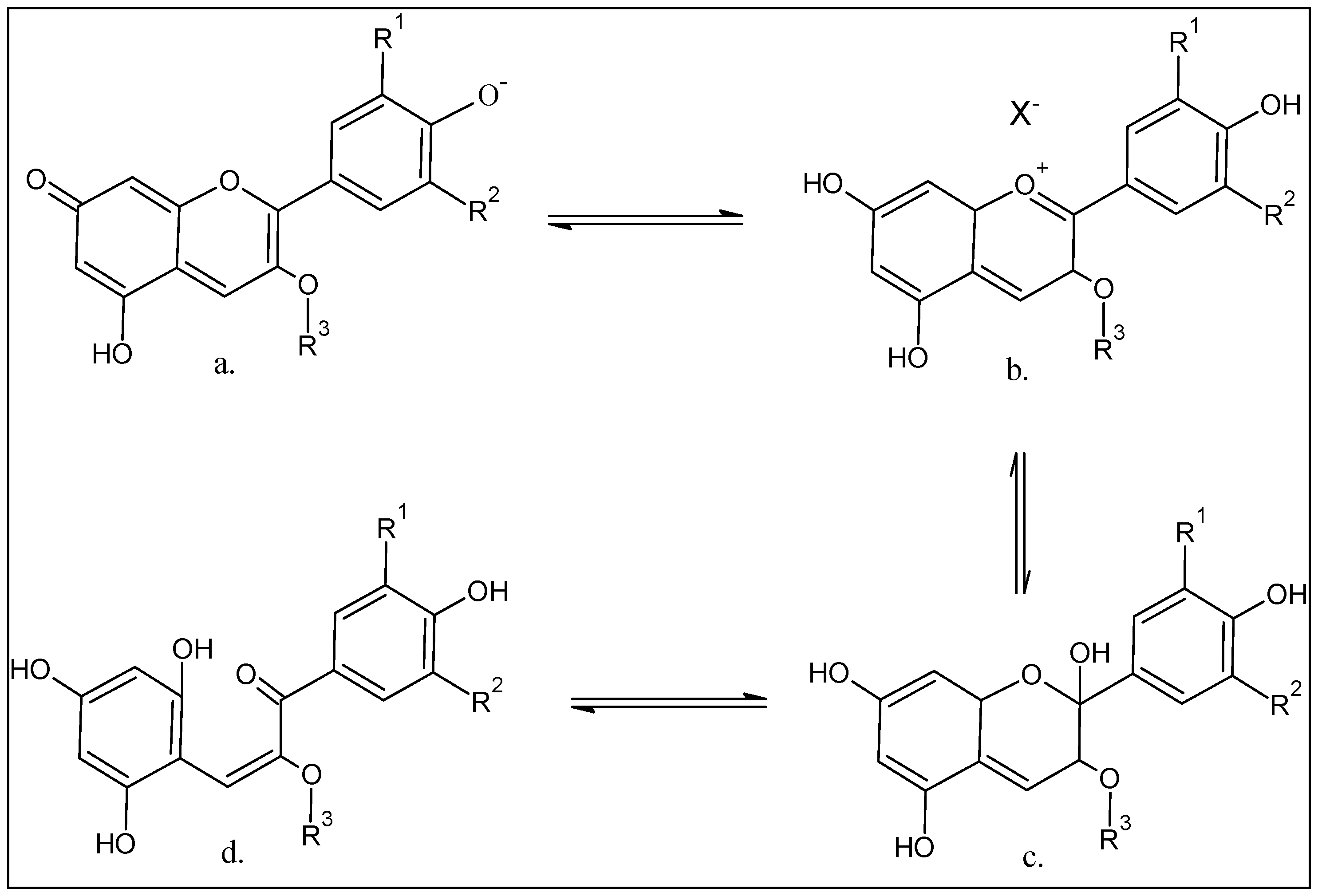
5. Anthocyanins Extraction, Purification, and Structure Determinations
6. Herbal Medicines Traditional Uses, Toxicity, Liver Disorders, and Anthocyanins
|
Plant’s Name |
Folklore Medicinal Uses, Other than Liver Disorders |
Plant Parts Used |
Major Identified Anthocyanins |
Refer |
|---|
9. Anthocyanins’ Suggestive Roles through Hepatic Biomarkers Regulation, and Biomechanistics Outlook
|
Anthocyanins |
Experimental Protocol |
Mode of Action |
Refer |
|---|
|
Plant’s Name |
Used Extracts, and/or Pure Compounds |
In Vivo/In Vitro Models and Bioactivity |
Major Anthocyanins |
Biomarkers, and Mode/Mechanism of Action |
Refer |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Hibiscus sabdariffa |
Hypertension, pyrexia |
Calyx, Epicalyx |
Cyanidin-3-O-β-glucoside, and delphinidin-3-glucoside |
|||||||
|
Cyanidin-3-O-β-glucoside |
In vivo CCl4-induced liver damage in mice and in vitro H2O2-induced oxidative stress in HepG2 cells apoptosis |
Enhance the antioxidant enzymes activities and upregulating Nrf2-antioxidant pathway. |
[156] | |||||||
|
Morus alba, and species |
Mulberry anthocyanins |
CCl4 (carbon tetrachloride), in vivo model. Hepatoprotection | [153] | |||||||
Cyanidin-3-O-β-glucoside | Decreased the ALT (alanine transaminase), AST (aspartate transaminase), hyaluronidase, hydroxyproline, and collagen type-III in the injured rats |
Cichorium intybus |
Delphinidin Inflammation |
In vitro H2O2Leaves |
-induced oxidative stress in HepG2 Cells | |||||
|
Ipomoea batatas L. | Anthocyanins rich purple sweet potato extract Enhance the expression of Nrf2 and promoted Nrf2 nuclear translocation. Increase expression of antioxidant protein HO-1 (Nrf2-related phase II enzyme heme oxygenase-1). Alleviate the reduction of Nrf2 protein levels and the accumulation of intracellular ROS levels in Nrf2 knockdown HepG2 cells.Cyanidin-3-O-(6″-malonyl-β-glucopyranoside) |
|||||||||
] | ||||||||||
CCl4, in vivo model. Hepatoprotection |
Peonidin-3-caffeoyl-feruloyl sophoroside-5-glucosid, peonidin 3-caffeoyl-p-hydroxy benzoyl sophoroside-5-glucoside, peonidin 3-dicaffeoyl sophoroside-5-glucoside |
Reduced the AST and ALT enzymes and MDA (malondialdehyde) level; Increased the SOD (superoxide dismutase), and GSH (glutathione) levels compared to the injured CCl4 administered group of animals |
Garcinia indica |
Mixture of cyanidin-3-O-β-glucoside, delphinidin-3-O-rutinoside, and malvidin-3-O-galactoside |
||||||
|
Oryza sativa | Male digestion, flatulence, and constipation. |
In vivo CCl4-induced human embryonic-liver (L-02) cells toxicity Fruits |
Anthocyanins rich black rice bran extract Reduce the percentage of hypo-diploid cells and decrease in caspase-3 protein expression Cyanidin-3-O-β-glucoside, and cyanidin-3-O-sambubioside |
CCl4, in vivo model. |
||||||
Hepatoprotection |
Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside | |||||||||
Reduced aminotransferase activity in serum, enhanced SOD and glutathione peroxidase (GSH-Px) activities, thiobarbituric acid reactive substances (TBARS), and 8-hydroxy-20-deoxyguanosine levels significantly decreased as compared to the CCl | 4 | intoxicated group. Liver histopathology confirmed pathological gains by ARBE administration |
Raphanus sativus |
Roots |
Pelargonidin derivatives |
|||||
|
Morus alba (Mulberry), & other species |
Cardiovascular diseases, nephritis, thirsty, constipation |
Fruits |
Cyanidin-3-O-rutinoside, cyanidin-3-O-glucoside |
|||||||
|
Cornus mas (cornelian cherry) |
Diabetes, diarrhea, fevers, rheumatic complains, skin diseases and urinary tract infections |
Fruits |
Cyanidin-3-O-galactoside, pelargonidin-3-O-galactoside, delphinidin-3-O-galactoside, cyanidin-3-O-rutinoside, pelargonidin-3-O-glucoside, pelargonidin-3-O-rutinoside, pegonidin-3-O-glucoside |
|||||||
|
Lannea microcarpa |
Scurvy, rickets and cough. |
Fruits |
Cyanidin-3-O-(2-O-β-D-xylopyranosyl)-β-D-galactopyranoside, and cyanidin-3-O-β-D-galactopyranoside. |
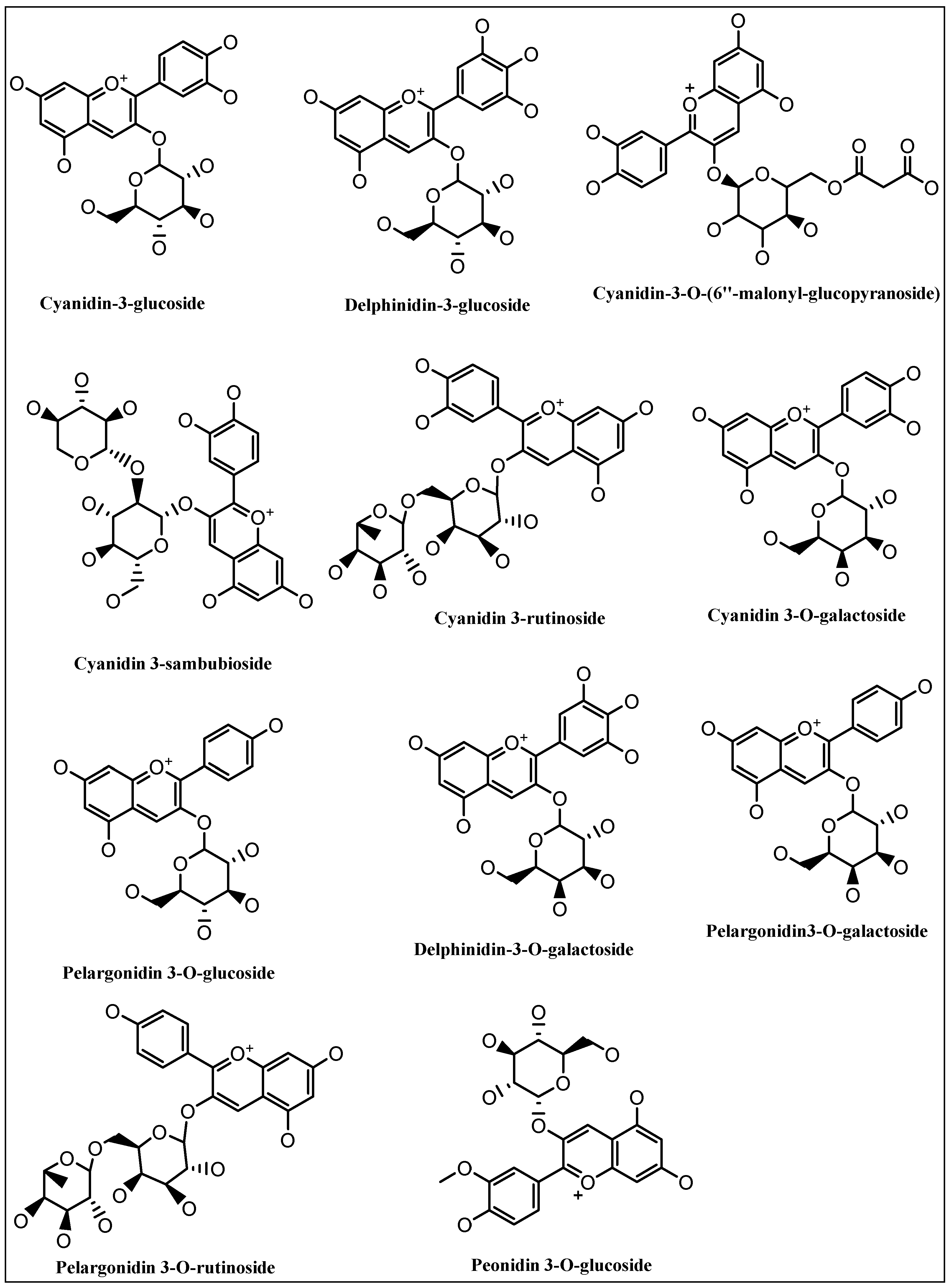
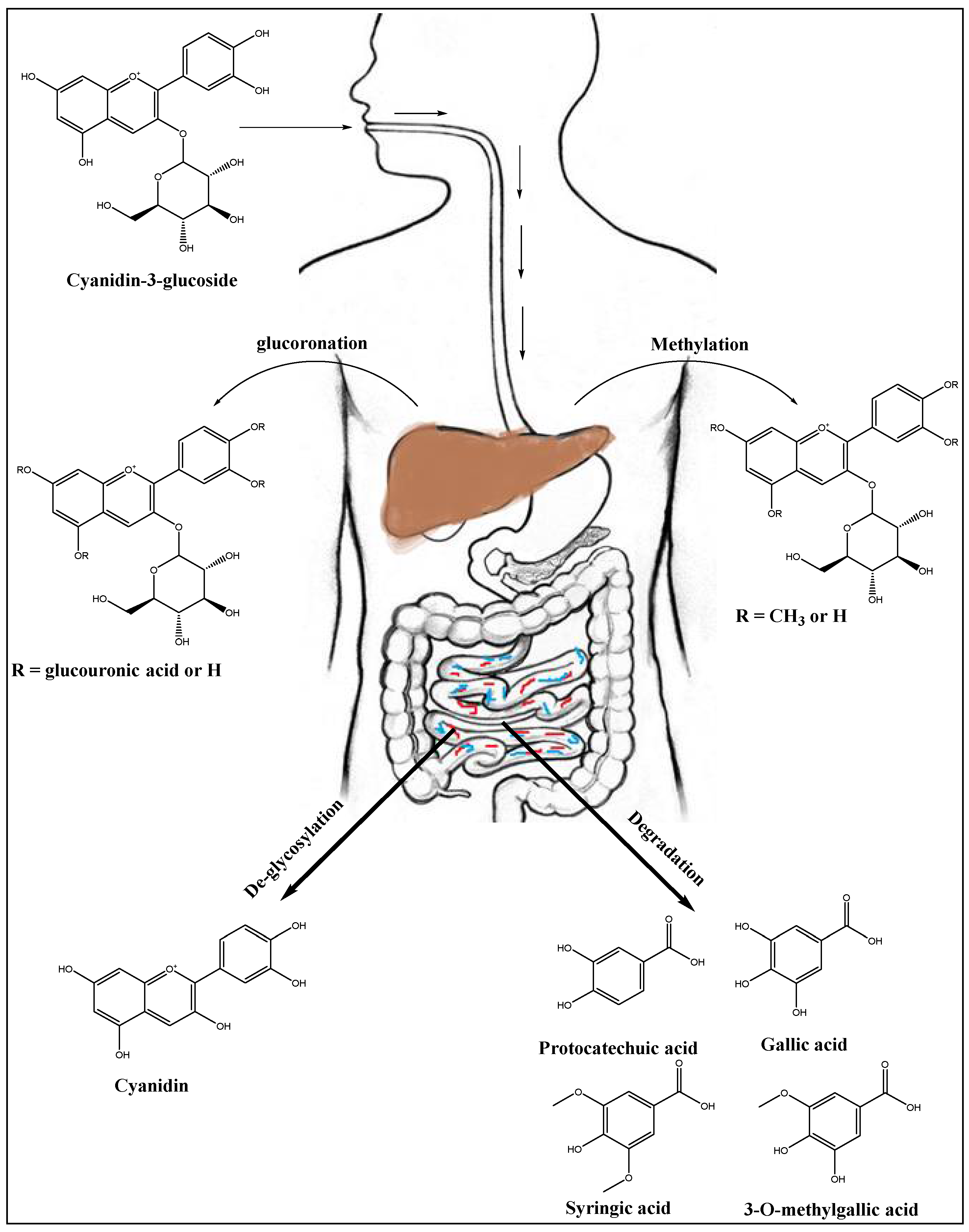
7. Anthocyanins’ Metabolism in Liver
8. Anthocyanins and Liver Disorders
Cyanidin-3-O-β-glucoside, and peonidin-3-O-glucoside | ||||
|
Ipomoea batatas |
In vitro human embryo non-malignant liver tissue cell line (L-02). Hepatoprotection |
Exhibited higher cell viability, decreased aminotransferase activity and enhanced cellular antioxidant status. Furthermore, Cy-3-G showed much stronger hepatoprotective activity than Pn-3-G at the same concentration. |
Anthocyanins rich fraction of purple sweet potato extract |
In vivo, ethanol, acetaminophen, and, CCl | |||||
4 | |||||
. | Hepatoprotection, and treatment |
3-O-(6-O-trans-caffeyt-2-O-~-glucopyranosyl/3-glucopyranoside)-5-O-glucosides of cyanidin, and peonidin |
Treatments of mice with anthocyanins fraction in dose dependent manner, and reduced the CYP2E1-dependent aniline hydroxylation, and CYP2E1 protein levels. Antioxidant effects on hepatic GSH level, and GSH S-transferase activity were up-regulated in FeCl2/ascorbate-induced lipid peroxidation in mouse liver homogenates, also showed superoxide radical scavenging activity. |
||
|
Hibiscus sabdariffa L. |
Anthocyanin-rich extract |
In vivo, thioacetamide (TAA)-induced hepatotoxicity. Hepatoprotection |
Cyanidine, delphinidin derivatives, cyanidin-3,5-O-di-glucoside, cyanidin-3-O-sophoroside-5-glucoside |
Reduced the serum levels of ALA, AST, and hepatic malondialdehyde, decreased hepatic inflammatory markers, including TNF-α, interleukin-6, and INF-γ, decreased the immuno-positivity of NF kappa-B, and CYP2E1 in liver tissues |
|
|
In vivo tert-BHP-induced cytotoxicity in rat |
|||||
|
CCl4 in vivo model. Hepatoprotection |
|||||
|
Aronia melanocarpa |
Fruit juice |
CCl4, N-nitroso diethyl amine, Paracetamol in vivo model. Hepatoprotection |
Cyanidin-3-O-galactoside, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside and cyanidin-3-O-β-glucoside |
Reduced necrotic changes in rat liver and inhibited increase of plasma AST and ALT activities, MDA formation induced by CCl4. Increased liver GSH contents. Decreased the activities of enzymatic markers of cytochrome P450, CYP1A1 and 1A2. |
|
|
Justicia spicigera |
Ethyl acetate fraction |
CCl4 in vivo model. Hepatoprotection |
Peonidin 3,5-O-di-glucoside, malvidin 3,5-O-di-glucoside, and petunidin 3,5-O-di-glucoside |
Improvement in liver function indices and oxidative stress markers. Increased SOD and GSH, and decreased MDA. |
|
|
Vaccinium sp. |
Berry pomace extract |
In vitro hepatic cell line HepG2 proliferation. Hepatic cells protection |
Procyanidin dimers |
Protects hepatic cells from oxidative damage. |
|
|
Solanum tuberosum L. |
Purple potato’s anthocyanins rich extract |
In vivo, alcoholic liver disease mouse model. Hepatoprotection |
Petunidin-3-coumaroyl-rutinoside-5-glucoside, peonidin-3-coumaroyl-rutinoside-5-glucoside, petunidin-3-O-glucoside, petunidin-3-rutinoside-5-glucoside, pelphinidin-3-coumaroyl-rutinoside-5-glucoside |
Higher levels of SOD and reduced GSH enzymes, reduction in formation of malondialdehyde, protected against alcohol-induced detrimental levels, maneuvered the activity of cytochrome P450 2E1 (CYP2E1) |
|
|
Ipomoea batatas |
Anthocyanin fraction |
Dimethyl nitrosamine-induced liver injury in rats. Hepatoprotection |
Cyanidin-3-O-β-glucoside chloride, malvidin-3-O-glucoside, pelargonidin-3-O-glucoside chloride, and peonidine-3-O-glucoside chloride |
Induced Nrf2 mediated antioxidant enzymes, and reduced the COX-2, and iNOS expressions, reduced inflammation through NF-KB inhibition |
|
|
Hibiscus sabdariffa |
Water extract, and anthocyanins |
Paracetamol-induced hepatotoxicity in rats. Hepatoprotection |
Anthocyanins |
Increased GSH and SOD levels, decreased ALT and AST |
|
|
Colocasia antiquorum |
Ethanolic extract |
Paracetamol, and CCl4 toxicated rats. Hepatoprotection |
Cyanidin-3-O-β-glucoside, pelargonidin-3-O-glucoside and cyanidin-3-O-rhamnoside |
Decreased ALT, and AST levels |
|
|
Vaccinium myrtillus and Ribes nigrum |
Anthocyanins-rich extracts |
Acetaminophen-induced hepatotoxicity in rats. Hepatoprotection |
Glycosides of cyanidin, peonidin, delphinidin, petunidin, and malvidin |
Normalized activities of glutamate oxaloacetate and glutamate pyruvate transaminase, prevented APAP-induced plasmatic and tissue alterations in biomarkers of oxidative stress |
|
|
Raphanus sativus L. (Red radish) |
Anthocyanins fraction |
CCl4 in vivo model. Hepatoprotection |
Pelargonidin derivatives |
Reversed the alteration of biochemical parameters to normal |
|
|
Raphanus sativus L. var. niger |
Fermented roots |
In vivo model for the methionine, and choline-deficient, diet-induced non-alcoholic fatty liver in mice. Hepatoprotection |
Pelargonidin derivatives |
Decreased lipids in 3T3-L1 adipocytes by downregulating adipogenic transcription factors, sterol regulatory element-binding protein 1c, CCAAT/enhancer-binding protein α, peroxisome proliferator-activated receptor γ, and lipid accumulation-related genes adipocyte protein-2, as well as fatty acid synthase. Decreased ALT, AST, TG levels. Deceased expression of iNO synthase, suppression of the inactivation of macrophages, and Kupffer cells in liver. Inhibition of α-smooth muscle actin, transforming growth factor β-1, and collagen type-I α-1 chain leading to reduced liver fibrosis. |
|
|
Raphanus sativus L. var. niger |
Aqueous extract of roots |
In vitro model in HepG2 cells. Hepatoprotection |
Pelargonidin derivatives |
Induced quinone reductase activity, and expression of multiple phase I, II detoxification enzymes in the HepG2 human hepatoma cell line |
|
|
Malvaviscus arboreus Cav |
Aerial parts extracts |
CCl4 in vivo model. Hepatoprotection |
Cyanidin-3-sambubioside |
EtOAc (ethyl acetate), and CH2Cl2 (Dichloromethane) extracts significantly reduced the liver injury in rats as indicated by the reduced levels of ALT, AST, ALP, TB, and MDA, comparatively the EtOAc fraction enhanced total antioxidant capacity of liver at the maximum. |
|
|
Cornus mas L. |
Anthocyanins rich fraction |
Lipid peroxidation, oxidative stress in the livers of cholesterol-fed rabbits |
Delphinidin 3-O-galactoside, cyanidin-3-O-galactoside, Cyanidin-3-O-robinobioside, pelargonidin-3-O-galactoside, pelargonidin-3-O-robinobioside, cyanidin, and pelargonidin. |
Decreased lipid peroxidation, decreased MDA levels, and reduced oxidative stress, an increase in liver GSH found. |
10. Anthocyanins Roles in Hepatocellular Longevity, Hepatic Carcinoma and Liver Cancer
References
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333.
- Varelis, P.; Melton, L.; Shahidi, F. Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 0128140453.
- Carle, R.; Schweiggert, R. Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Woodhead Publishing: Sawston, UK, 2016; ISBN 0081003927.
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075.
- Fanning, K.; Edwards, D.; Netzel, M.; Stanley, R.; Netzel, G.; Russell, D.; Topp, B. Increasing anthocyanin content in Queen Garnet plum and correlations with in-field measures. Acta Hortic. 2013, 985, 97–104.
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856.
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030.
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Shimoi, K.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462.
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500.
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J. Agric. Food Chem. 2007, 55, 10832–10838.
- Hiemori, M.; Koh, E.; Mitchell, A.E. Influence of cooking on anthocyanins in black rice (Oryza sativa L. japonica var. SBR). J. Agric. Food Chem. 2009, 57, 1908–1914.
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400.
- Herrera-Sotero, M.Y.; Cruz-Hernández, C.D.; Trujillo-Carretero, C.; Rodríguez-Dorantes, M.; García-Galindo, H.S.; Chávez-Servia, J.L.; Oliart-Ros, R.M.; Guzmán-Gerónimo, R.I. Antioxidant and antiproliferative activity of blue corn and tortilla from native maize. Chem. Cent. J. 2017, 11, 110.
- Lieberman, S. The antioxidant power of purple corn: A research review. Altern. Complement. Ther. 2007, 13, 107–110.
- Li, C.-Y.; Kim, H.-W.; Won, S.R.; Min, H.-K.; Park, K.-J.; Park, J.-Y.; Ahn, M.-S.; Rhee, H.-I. Corn husk as a potential source of anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416.
- Munoz-Espada, A.C.; Wood, K.V.; Bordelon, B.; Watkins, B.A. Anthocyanin quantification and radical scavenging capacity of Concord, Norton, and Marechal Foch grapes and wines. J. Agric. Food Chem. 2004, 52, 6779–6786.
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531.
- de Moura, C.; dos Reis, A.S.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Pereira, C.; Carpes, S.T. Optimization of phenolic compounds extraction with antioxidant activity from açaí, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018, 30, 180–189.
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779.
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A 2013, 1281, 38–45.
- Scheuermann, E.; Seguel, I.; Montenegro, A.; Bustos, R.O.; Hormazábal, E.; Quiroz, A. Evolution of aroma compounds of murtilla fruits (Ugni molinae Turcz) during storage. J. Sci. Food Agric. 2008, 88, 485–492.
- Pennington, J.A.T.; Fisher, R.A. Food component profiles for fruit and vegetable subgroups. J. Food Compos. Anal. 2010, 23, 411–418.
- Lachman, J.; Orsák, M.; Pivec, V. Antioxidant contents and composition in some vegetables and their role in human nutrition. Hortic. Sci. 2000, 27, 65–78.
- Brito, A.; Areche, C.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin characterization, total phenolic quantification and antioxidant features of some Chilean edible berry extracts. Molecules 2014, 19, 10936–10955.
- Lees, D.-H.; Francis, F.J. Standardization of pigment analyses in cranberries. Hort Sci. 1972, 7, 83–84.
- De Rosso, V.V.; Hillebrand, S.; Montilla, E.C.; Bobbio, F.O.; Winterhalter, P.; Mercadante, A.Z. Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açai (Euterpe oleracea Mart.) by HPLC–PDA–MS/MS. J. Food Compos. Anal. 2008, 21, 291–299.
- Ella Missang, C.; Guyot, S.; Renard, C.M.G.C. Flavonols and anthocyanins of bush butter, Dacryodes edulis (G. Don) HJ Lam, fruit. Changes in their composition during ripening. J. Agric. Food Chem. 2003, 51, 7475–7480.
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517.
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500.
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of anthocyanin content and profile throughout fruit development and ripening of highbush blueberry cultivars grown at two different altitudes. Front. Plant Sci. 2019, 10, 1045.
- Mannino, G.; Gentile, C.; Maffei, M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry 2019, 160, 40–47.
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212.
- Gould, K.S.; Lister, C. Flavonoid functions in plants. In Flavonoids Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2006; pp. 397–441.
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9.
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361.
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669.
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762.
- Huang, Z.; Wang, Q.; Xia, L.; Hui, J.; Li, J.; Feng, Y.; Chen, Y. Preliminarily exploring of the association between sugars and anthocyanin accumulation in apricot fruit during ripening. Sci. Hortic. 2019, 248, 112–117.
- Aza-Gonzalez, C.; Herrera-Isidrón, L.; Núñez-Palenius, H.G.; De La Vega, O.M.; Ochoa-Alejo, N. Anthocyanin accumulation and expression analysis of biosynthesis-related genes during chili pepper fruit development. Biol. Plant. 2013, 57, 49–55.
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871.
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809.
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187.
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428.
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915.
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Debski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Veg. Crop. Res. Bull. 2008, 68, 5–22.
- He, K.; Li, X.; Chen, X.; Ye, X.; Huang, J.; Jin, Y.; Li, P.; Deng, Y.; Jin, Q.; Shi, Q. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J. Ethnopharmacol. 2011, 137, 1135–1142.
- Sims, C.A.; Morris, J.R. A comparison of the color components and color stability of red wine from Noble and Cabernet Sauvignon at various pH levels. Am. J. Enol. Vitic. 1985, 36, 181–184.
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982.
- Rein, M. Copigmentation Reactions and Color Stability of Berry Anthocyanins; University of Helsinki: Helsinki, Finland, 2005.
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470.
- Dincheva, I.; Badjakov, I. Assesment of the anthocyanin variation in bulgarian bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.). Int. J. Med. Pharm. Sci. 2016, 6, 39–50.
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MSn. Food Chem. 2017, 221, 1883–1894.
- Nankar, A.N.; Dungan, B.; Paz, N.; Sudasinghe, N.; Schaub, T.; Holguin, F.O.; Pratt, R.C. Quantitative and qualitative evaluation of kernel anthocyanins from southwestern United States blue corn. J. Sci. Food Agric. 2016, 96, 4542–4552.
- Sang, J.; Sang, J.; Ma, Q.; Hou, X.; Li, C. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017, 218, 386–395.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharm. Res. 2015, 38, 2143–2152.
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203.
- Da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; da Silva, C.d.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470.
- Ongkowijoyo, P.; Luna-Vital, D.A.; de Mejia, E.G. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126.
- Jampani, C.; Naik, A.; Raghavarao, K. Purification of anthocyanins from jamun (Syzygium cumini L.) employing adsorption. Sep. Purif. Technol. 2014, 125, 170–178.
- Heinonen, J.; Farahmandazad, H.; Vuorinen, A.; Kallio, H.; Yang, B.; Sainio, T. Extraction and purification of anthocyanins from purple-fleshed potato. Food Bioprod. Process. 2016, 99, 136–146.
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 338–343.
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796.
- Ying, L.; Jia-Ying, L.; Jing, L.; Mi-Lu, L.; Zhong-Hua, L. Preparative separation of anthocyanins from purple sweet potatoes by high-speed counter-current chromatography. Chin. J. Anal. Chem. 2011, 39, 851–856.
- Vatai, T.; Škerget, M.; Knez, Ž.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36.
- Lao, F.; Giusti, M.M. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: Method comparison and correlation. Food Anal. Methods 2016, 9, 1367–1380.
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278.
- Fuleki, T.; Francis, F.J. Quantative methods for analysis. 2. Determination of total anthocyanin and degeadition index in cranberries. J. Food Sci. 1969, 33, 78–83.
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, phenolics, and color of Cabernet franc, Merlot, and Pinot noir wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017.
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241.
- King, J.W.; Grabiel, R.D.; Wightman, J.D. Subcritical water extraction of anthocyanins from fruit berry substrates. In Proceedings of the 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003; Volume 1, pp. 28–30.
- Ju, Z.; Howard, L.R. Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276.
- Schwarz, M.; Hillebrand, S.; Habben, S.; Degenhardt, A.; Winterhalter, P. Application of high-speed countercurrent chromatography to the large-scale isolation of anthocyanins. Biochem. Eng. J. 2003, 14, 179–189.
- Liu, Y.; Liu, J.; Chen, X.; Liu, Y.; Di, D. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 2010, 123, 1027–1034.
- Petersson, E.V. Analysis of Acrylamide and Anthocyanins in Foods: Extraction Optimization for Challenging Analytes; Uppsala University: Uppsala, Sweden, 2009.
- Brauch, J.E.; Reuter, L.; Conrad, J.; Vogel, H.; Schweiggert, R.M.; Carle, R. Characterization of anthocyanins in novel Chilean maqui berry clones by HPLC–DAD–ESI/MSn and NMR-spectroscopy. J. Food Compos. Anal. 2017, 58, 16–22.
- Stein-Chisholm, R.E.; Beaulieu, J.C.; Grimm, C.C.; Lloyd, S.W. LC–MS/MS and UPLC–UV evaluation of anthocyanins and anthocyanidins during rabbiteye blueberry juice processing. Beverages 2017, 3, 56.
- Barnes, J.S.; Schug, K.A. Structural characterization of cyanidin-3,5-diglucoside and pelargonidin-3,5-diglucoside anthocyanins: Multi-dimensional fragmentation pathways using high performance liquid chromatography-electrospray ionization-ion trap-time of flight mass spectrometr. Int. J. Mass Spectrom. 2011, 308, 71–80.
- Marković, J.M.D.; Baranac, J.M.; Brdarić, T.P. Electronic and infrared vibrational analysis of cyanidin–quercetin copigment complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62, 673–680.
- Mateus, N.; Silva, A.M.S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; de Freitas, V. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry. J. Agric. Food Chem. 2002, 50, 2110–2116.
- Andersen, Ø.M.; Fossen, T. Characterization of anthocyanins by NMR. Curr. Protoc. Food Anal. Chem. 2003, 9, F1–F4.
- Stickel, F.; Egerer, G.; Seitz, H.K. Hepatotoxicity of botanicals. Public Health Nutr. 2000, 3, 113–124.
- Stedman, C. Herbal hepatotoxicity. Semin. Liver Dis. 2002, 22, 195–206.
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373.
- Zheng, E.; Sandhu, N.; Navarro, V. Drug-induced liver injury secondary to herbal and dietary supplements. Clin. Liver Dis. 2020, 24, 141–155.
- Hartleb, M.; Biernat, L.; Kochel, A. Drug-induced liver damage—A three-year study of patients from one gastroenterological department. Med. Sci. Monit. 2002, 8, CR292–CR296.
- Voican, C.S.; Corruble, E.; Naveau, S.; Perlemuter, G. Antidepressant-induced liver injury: A review for clinicians. Am. J. Psychiatry 2014, 171, 404–415.
- Park, S.H.; Ishino, R. Liver injury associated with antidepressants. Curr. Drug Saf. 2013, 8, 207–223.
- Miele, L.; Liguori, A.; Marrone, G.; Biolato, M.; Araneo, C.; Vaccaro, F.G.; Gasbarrini, A.; Grieco, A. Fatty liver and drugs: The two sides of the same coin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 86–94.
- Amacher, D.E.; Chalasani, N. Drug-induced hepatic steatosis. Semin. Liver Dis 2014, 34, 205–214.
- WebMD. Available online: https://www.webmd.com/search/search_results/default.aspx?query=liver diseases (accessed on 24 December 2021).
- Mataya, L.; Patel, N.; Azzam, R.K. Autoimmune liver diseases in children. Pediatr. Ann. 2018, 47, e452–e457.
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735.
- Viveiros, K. The Role of Life Style Modifications in Comprehensive Non-Alcoholic Fatty Liver Disease Treatment. Clin. Liver Dis. 2021, 17, 11.
- Dhiman, R.K.; Chawla, Y.K. Herbal medicines for liver diseases. Dig. Dis. Sci. 2005, 50, 1807–1812.
- Sharma, R. Herbal Home Remedies; Lotus Press: Wisconsin, WA, USA, 2006; ISBN 8183820549.
- Konczak, I.; Zhang, W. Anthocyanins—More than nature’s colours. J. Biomed. Biotechnol. 2004, 2004, 239.
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin-and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10.
- Wang, C.-J.; Wang, J.-M.; Lin, W.-L.; Chu, C.-Y.; Chou, F.-P.; Tseng, T.-H. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem. Toxicol. 2000, 38, 411–416.
- Mulabagal, V.; Wang, H.; Ngouajio, M.; Nair, M.G. Characterization and quantification of health beneficial anthocyanins in leaf chicory (Cichorium intybus) varieties. Eur. Food Res. Technol. 2009, 230, 47–53.
- Chaves-López, C.; Yeimmy, P.-R.; Molina Hernandez, J.B.; Delgado Ospina, J.; Tovar, C.D.G.; Paparella, A. Anthocyanins in Folk Medicine: Local Traditions, Sources, Compounds and Related Aspects. In Anthocyanins; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; p. 141.
- Dash, R.N.; Habibuddin, M.; Baruah, D.B. Anthocyanins fraction of red radish (Raphanus sativus L.) protects hepatic damage induced by carbon tetrachloride in albino rats. J. Exp. Integr. Med. 2013, 3, 43–50.
- Huo, Y. Mulberry Cultivation and Utilization in China; FAO Animal Production and Health Papers; FAO: Rome, Italy, 2000; pp. 11–44.
- Bagachi, A.; Semwal, A.; Bharadwaj, A. Traditional uses, phytochemistry and pharmacology of Morus alba Linn.: A review. J. Med. Plants Res. 2013, 7, 461–469.
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690.
- Ajiboye, T.O.; Raji, H.O.; Muritala, H.F.; Ojewuyi, O.B.; Yakubu, M.T. Anthocyanin extract of Lannea microcarpa fruits stall oxidative rout associated with aflatoxin B1 hepatocarcinogenesis. Food Biosci. 2013, 4, 58–67.
- Awad, N.E.; Abdelkawy, M.A.; Hamed, M.A.; Souleman, A.M.A.; Abdelrahman, E.H.; Ramadan, N.S. Antioxidant and hepatoprotective effects of Justicia spicigera ethyl acetate fraction and characterization of its anthocyanin content. Int. J. Pharm. Pharm. Sci. 2015, 7, 91–96.
- Ezzat, S.M.; Salama, M.M.; Seif el-Din, S.H.; Saleh, S.; El-Lakkany, N.M.; Hammam, O.A.; Salem, M.B.; Botros, S.S. Metabolic profile and hepatoprotective activity of the anthocyanin-rich extract of Hibiscus sabdariffa calyces. Pharm. Biol. 2016, 54, 3172–3181.
- Hou, F.; Zhang, R.; Zhang, M.; Su, D.; Wei, Z.; Deng, Y.; Zhang, Y.; Chi, J.; Tang, X. Hepatoprotective and antioxidant activity of anthocyanins in black rice bran on carbon tetrachloride-induced liver injury in mice. J. Funct. Foods 2013, 5, 1705–1713.
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089.
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271.
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.E.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712.
- Tsuda, T.; Horio, F.; Osawa, T. The role of anthocyanins as an antioxidant under oxidative stress in rats. Biofactors 2000, 13, 133–139.
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary anthocyanins and insulin resistance: When food becomes a medicine. Nutrients 2017, 9, 1111.
- Ávila, M.; Hidalgo, M.; Sánchez-Moreno, C.; Pelaez, C.; Requena, T.; de Pascual-Teresa, S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461.
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205.
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142.
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942.
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The role of anthocyanins as antidiabetic agents: From molecular mechanisms to in vivo and human studies. J. Physiol. Biochem. 2021, 77, 109–131.
- Ormazabal, P.; Scazzocchio, B.; Varì, R.; Santangelo, C.; D’Archivio, M.; Silecchia, G.; Iacovelli, A.; Giovannini, C.; Masella, R. Effect of protocatechuic acid on insulin responsiveness and inflammation in visceral adipose tissue from obese individuals: Possible role for PTP1B. Int. J. Obes. 2018, 42, 2012–2021.
- Habib, S.A.; Suddek, G.M.; Rahim, M.A.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485.
- Lin, W.-L.; Hsieh, Y.-J.; Chou, F.-P.; Wang, C.-J.; Cheng, M.-T.; Tseng, T.-H. Hibiscus protocatechuic acid inhibits lipopolysaccharide-induced rat hepatic damage. Arch. Toxicol. 2003, 77, 42–47.
- Owumi, S.E.; Ajijola, I.J.; Agbeti, O.M. Hepatorenal protective effects of protocatechuic acid in rats administered with anticancer drug methotrexate. Hum. Exp. Toxicol. 2019, 38, 1254–1265.
- Fu, R.; Zhou, J.; Wang, R.; Sun, R.; Feng, D.; Wang, Z.; Zhao, Y.; Lv, L.; Tian, X.; Yao, J. Protocatechuic acid-mediated miR-219a-5p activation inhibits the p66shc oxidant pathway to alleviate alcoholic liver injury. Oxid. Med. Cell. Longev. 2019, 2019, 3527809.
- Neshat, S.Y.; Quiroz, V.M.; Wang, Y.; Tamayo, S.; Doloff, J.C. Liver Disease: Induction, Progression, Immunological Mechanisms, and Therapeutic Interventions. Int. J. Mol. Sci. 2021, 22, 6777.
- Fausto, N. Liver regeneration. J. Hepatol. 2000, 32, 19–31.
- Sánchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860.
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124.
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; Van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37.
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4234061.
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141.
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246.
- Castellani, R.; Hirai, K.; Aliev, G.; Drew, K.L.; Nunomura, A.; Takeda, A.; Cash, A.D.; Obrenovich, M.E.; Perry, G.; Smith, M.A. Role of mitochondrial dysfunction in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 357–360.
- Yusuf, M.; Khan, M.; Robaian, M.A.; Khan, R.A. Biomechanistic insights into the roles of oxidative stress in generating complex neurological disorders. Biol. Chem. 2018, 399, 305–319.
- Cichoż-Lach, H. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082.
- Wu, X.; Kang, J.; Xie, C.; Burris, R.; Ferguson, M.E.; Badger, T.M.; Nagarajan, S. Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J. Nutr. 2010, 140, 1628–1632.
- Feagins, L.A.; Flores, A.; Arriens, C.; Park, C.; Crook, T.; Reimold, A.; Brown, G. Nonalcoholic fatty liver disease: A potential consequence of tumor necrosis factor-inhibitor therapy. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1154.
- Coffin, C.S.; Fraser, H.F.; Panaccione, R.; Ghosh, S. Liver diseases associated with anti-tumor necrosis factor-alpha (TNF-α) use for inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 479–484.
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, UK, 2018; ISBN 1786393255.
- Aboelsoud, N.H. Herbal medicine in ancient Egypt. J. Med. Plants Res. 2010, 4, 82–86.
- Jain, S.K. Ethnobotany and research in medicinal plants in India. Ethnobot. Search New Drugs 1994, 185, 153–168.
- Mohammed, S.A.A.; Eldeeb, H.M.; Mohammed, H.A.; Al-Omar, M.S.; Almahmoud, S.A.; El-Readi, M.Z.; Ragab, E.A.; Sulaiman, G.M.; Aly, M.S.A.; Khan, R.A. Roles of Suaeda vermiculata Aqueous-Ethanolic Extract, Its Subsequent Fractions, and the Isolated Compounds in Hepatoprotection against Paracetamol-Induced Toxicity as Compared to Silymarin. Oxid. Med. Cell. Longev. 2021, 2021, 6174897.
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. Chapter 6—Sweet Potato Anthocyanins. In Sweet Potato Processing Technology; Mu, T., Sun, H., Zhang, M., Wang, C., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 279–355. ISBN 978-0-12-812871-8.
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxid. Med. Cell. Longev. 2020, 2020, 2735790.
- Jia, Y.; Kim, J.-Y.; Jun, H.; Kim, S.-J.; Lee, J.-H.; Hoang, M.H.; Kim, H.S.; Chang, H.I.; Hwang, K.-Y.; Um, S.-J. Cyanidin is an agonistic ligand for peroxisome proliferator-activated receptor-alpha reducing hepatic lipid. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2013, 1831, 698–708.
- Chang, J.-J.; Hsu, M.-J.; Huang, H.-P.; Chung, D.-J.; Chang, Y.-C.; Wang, C.-J. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 2013, 61, 6069–6076.
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.-H.; Jung, K.O.; Jung, K.H.; Kwon, K.; Jeong, T.C.; Chung, Y.C. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate–activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906.
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic. Biol. Med. 2012, 52, 314–327.
- Pei, L.; Wan, T.; Wang, S.; Ye, M.; Qiu, Y.; Jiang, R.; Pang, N.; Huang, Y.; Zhou, Y.; Jiang, X. Cyanidin-3-O-β-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 2018, 105, 625–632.
- Castera, L. Steatosis, insulin resistance and fibrosis progression in chronic hepatitis C. Minerva Gastroenterol. Dietol. 2006, 52, 125–134.
- Powell, E.E.; Jonsson, J.R.; Clouston, A.D. Steatosis: Co-factor in other liver diseases. Hepatology 2005, 42, 5–13.
- Yu, L.; Zhang, S.; Zhao, X.; Ni, H.; Song, X.; Wang, W.; Yao, L.; Zhao, X.; Fu, Y. Cyanidin-3-glucoside protects liver from oxidative damage through AMPK/Nrf2 mediated signaling pathway in vivo and in vitro. J. Funct. Foods 2020, 73, 104148.
- Xu, J.; Zhang, Y.; Ren, G.; Yang, R.; Chen, J.; Xiang, X.; Qin, H.; Chen, J. Inhibitory effect of delphinidin on oxidative stress induced by H2O2 in HepG2 cells. Oxid. Med. Cell. Longev. 2020, 2020, 4694760.
- Chen, J.; Zhao, Y.; Tao, X.; Zhang, M.; Sun, A. Protective effect of blueberry anthocyanins in a CCL4-induced liver cell model. LWT—Food Sci. Technol. 2015, 60, 1105–1112.
- Li, Y.; Yang, Z.; Jia, S.; Yuan, K. Protective effect and mechanism of action of mulberry marc anthocyanins on carbon tetrachloride-induced liver fibrosis in rats. J. Funct. Foods 2016, 24, 595–601.
- Wang, L.; Zhao, Y.; Zhou, Q.; Luo, C.-L.; Deng, A.-P.; Zhang, Z.-C.; Zhang, J.-L. Characterization and hepatoprotective activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8). J. Food Drug Anal. 2017, 25, 607–618.
- Choi, J.H.; Choi, C.Y.; Lee, K.J.; Hwang, Y.P.; Chung, Y.C.; Jeong, H.G. Hepatoprotective effects of an anthocyanin fraction from purple-fleshed sweet potato against acetaminophen-induced liver damage in mice. J. Med. Food 2009, 12, 320–326.
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral hepatoprotective ability evaluation of purple sweet potato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014, 69, 539–548.
- Onyesom, I.; Mordi, J.; Opajobi, A.O.; Esume, C.O. Hepatoprotective Potentials of Hibiscus rosasinensis Petal anthocyanin Extracts against Carbon tetrachloride-Induced Acute Liver Damage in Wistar Rats. Sudan J. Med. Sci. 2008, 3, 33–36.
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201.
- Valcheva-Kuzmanova, S. Comparative Study of the Protective Effect of Fruit Juice and Quercetin in a Model of Paracetamol-Induced Hepatotoxicity in Rats. J. Biomed. Clin. Res. 2015, 8, 118–123.
- Krajka-Kuźniak, V.; Szaefer, H.; Ignatowicz, E.; Adamska, T.; Oszmiański, J.; Baer-Dubowska, W. Effect of Chokeberry (Aronia melanocarpa) Juice on the Metabolic Activation and Detoxication of Carcinogenic N-Nitrosodiethylamine in Rat Liver. J. Agric. Food Chem. 2009, 57, 5071–5077.
- Muceniece, R.; Klavins, L.; Kviesis, J.; Jekabsons, K.; Rembergs, R.; Saleniece, K.; Dzirkale, Z.; Saulite, L.; Riekstina, U.; Klavins, M. Antioxidative, hypoglycaemic and hepatoprotective properties of five Vaccinium spp. berry pomace extracts. J. Berry Res. 2019, 9, 267–282.
- Jiang, Z.; Chen, C.; Wang, J.; Xie, W.; Wang, M.; Li, X.; Zhang, X. Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 2016, 70, 45–53.
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99.
- Ali, B.H.; Mousa, H.M.; El-Mougy, S. The effect of a water extract and anthocyanins of Hibiscus sabdariffa L. on paracetamol-induced hepatoxicity in rats. Phyther. Res. 2003, 17, 56–59.
- Tuse, T.A.; Harle, U.N.; Bore, V.V. Hepatoprotective activity of Colocasia antiquorum against experimentally induced liver injury in rats. Malyasian J. Pharm. Sci. 2009, 7, 99–112.
- Cristani, M.; Speciale, A.; Mancari, F.; Arcoraci, T.; Ferrari, D.; Fratantonio, D.; Saija, A.; Cimino, F.; Trombetta, D. Protective activity of an anthocyanin-rich extract from bilberries and blackcurrants on acute acetaminophen-induced hepatotoxicity in rats. Nat. Prod. Res. 2016, 30, 2845–2849.
- Ahn, M.; Kim, J.; Choi, Y.; Ekanayake, P.; Chun, J.; Yang, D.; Kim, G.-O.; Shin, T. Fermented black radish (Raphanus sativus L. var. niger) attenuates methionine and choline deficient diet-induced nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2019, 7, 3327–3337.
- Hanlon, P.R.; Robbins, M.G.; Hammon, L.D.; Barnes, D.M. Aqueous extract from the vegetative portion of Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzyme expression in HepG2 cells. J. Funct. Foods 2009, 1, 356–365.
- Abdelhafez, O.H.; Fawzy, M.A.; Fahim, J.R.; Desoukey, S.Y.; Krischke, M.; Mueller, M.J.; Abdelmohsen, U.R. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 2018, 13, e0202362.
- Sozański, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513.
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J. Anthocyanins delay ageing-related degenerative changes in the liver. Plant Foods Hum. Nutr. 2017, 72, 425–431.
- Lin, B.; Gong, C.; Song, H.; Cui, Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243.
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485.
- Fisher, C.D.; Lickteig, A.J.; Augustine, L.M.; Ranger-Moore, J.; Jackson, J.P.; Ferguson, S.S.; Cherrington, N.J. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab. Dispos. 2009, 37, 2087–2094.
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Baylor Univ. Med. Cent. Proc. 2000, 13, 421–423.
- Albertolle, M.E.; Phan, T.T.N.; Pozzi, A.; Guengerich, F.P. Sulfenylation of human liver and kidney microsomal cytochromes P450 and other drug-metabolizing enzymes as a response to redox alteration. Mol. Cell. Proteom. 2018, 17, 889–900.
- Hanioka, N.; Jinno, H.; Nishimura, T.; Ando, M. Changes in cytochrome P450 enzymes by 1, 1-dichloroethylene in rat liver and kidney. Arch. Toxicol. 1997, 72, 9–16.
- Albertolle, M.E.; Kim, D.; Nagy, L.D.; Yun, C.-H.; Pozzi, A.; Savas, Ü.; Johnson, E.F.; Guengerich, F.P. Heme–thiolate sulfenylation of human cytochrome P450 4A11 functions as a redox switch for catalytic inhibition. J. Biol. Chem. 2017, 292, 11230–11242.
- Tomankova, V.; Anzenbacher, P.; Anzenbacherova, E. Effects of obesity on liver cytochromes P450 in various animal models. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2017, 161, 144–151.
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057.
