Protons and carbon ions (hadrons) have useful properties for the treatments of patients affected by oncological pathologies. They are more precise than conventional X-rays and possess radiobiological characteristics suited for treating radio-resistant or inoperable tumours. This paper gives an overview of the status of hadron therapy around the world. It focusses on the Italian National Centre for Oncological Hadron therapy (CNAO), introducing operation procedures, system performance, expansion projects, methodologies and modelling to build individualized treatments. There is growing evidence that supports safety and effectiveness of hadron therapy for a variety of clinical situations. However, there is still a lack of high-level evidence directly comparing hadron therapy with modern conventional radiotherapy techniques.
- hadron therapy
- carbon ions therapy
- proton therapy
- medical synchrotron
- gantry
1. Introduction
Rationale and Diffusion of Hadron Therapy in the World
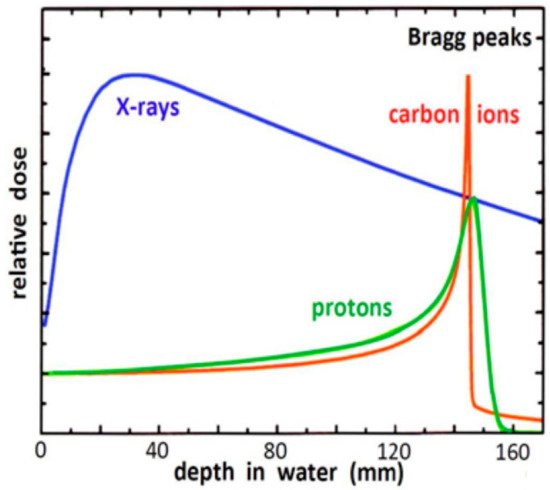

2.Hadron Therapy Achievements and Challenges
2.1. Pre-Clinical Radiobiology Research
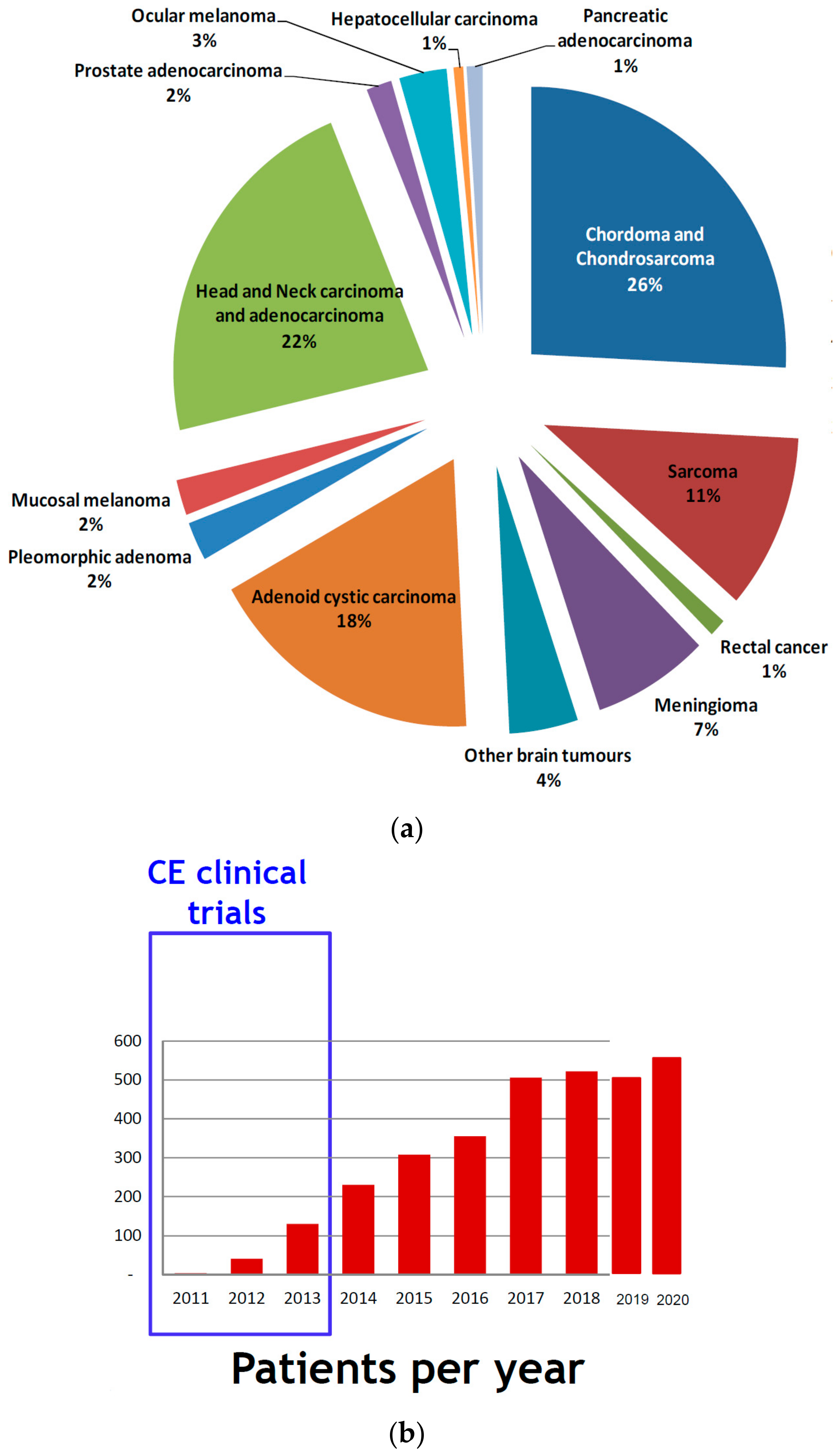
2.2. Clinical Activities: Pathologies and Results
-
chordomas and chondrosarcomas (of the skull base and of the spine);
-
meningioma;
-
brain tumours (trunk);
-
adenoid cystic carcinomas of the salivary glands;
-
orbit tumours including eye melanoma;
-
sino-nasal carcinomas;
-
soft tissue and bone sarcomas (all sites);
-
recurrent tumours (retreatment);
-
patients with immunological disorders;
-
paediatric solid tumours.
- chordomas and chondrosarcomas (of the skull base and of the spine);
- meningioma;
- brain tumours (trunk);
- adenoid cystic carcinomas of the salivary glands;
- orbit tumours including eye melanoma;
- sino-nasal carcinomas;
- soft tissue and bone sarcomas (all sites);
- recurrent tumours (retreatment);
- patients with immunological disorders;
- paediatric solid tumours.
2.3. Skull Base Chordoma and Chondrosarcoma
2.4. Head-and-Neck Tumours
2.5. Malignant Mucosal Melanoma
2.6. Clinical Research Trials
-
PIOPPO (preoperative treatment of borderline operable pancreatic adenocarcinomas with chemotherapy and radiotherapy with carbon ions) [96]: a phase 2 study, to evaluate the neo-adjuvant combination approach with chemotherapy followed by short-course carbon-ion radiotherapy for borderline pancreatic adenocarcinomas [97];
-
INSIDE: an experimental observational real-time live study of the particle range. This study is aimed at the early identification of potential morphological modifications of the target or of the adjacent areas, which might cause an anomaly in the dose distribution.
- PIOPPO (preoperative treatment of borderline operable pancreatic adenocarcinomas with chemotherapy and radiotherapy with carbon ions) [44]: a phase 2 study, to evaluate the neo-adjuvant combination approach with chemotherapy followed by short-course carbon-ion radiotherapy for borderline pancreatic adenocarcinomas [45];
- CYCLE (carbon ion radiation therapy in the treatment of mucosal melanomas of the female lower genital tract): a phase 2 study to test the efficacy and the tolerability of carbon-ion treatments of unresectable gynaecological mucosal melanomas;
-
CYCLOPS (Phase II clinical study on the re-irradiation of lateral pelvic recurrences of gynecological malignancies) a phase 2 study, to evaluate the efficacy and tolerability of carbon-ion re-irradiation for not central relapses of gynaecological neoplasms at the edge of the previous photon beam radiotherapy;
- CYCLE (carbon ion radiation therapy in the treatment of mucosal melanomas of the female lower genital tract): a phase 2 study to test the efficacy and the tolerability of carbon-ion treatments of unresectable gynaecological mucosal melanomas;
- 4D-MRI (guidance for organ motion management in particle treatments of thoraco-abdominal tumours): a clinical trial to study the organ motion of thoraco-abdominal neoplasms through 4D MRI;
- CYCLOPS (Phase II clinical study on the re-irradiation of lateral pelvic recurrences of gynecological malignancies) a phase 2 study, to evaluate the efficacy and tolerability of carbon-ion re-irradiation for not central relapses of gynaecological neoplasms at the edge of the previous photon beam radiotherapy;
- 4D-MRI (guidance for organ motion management in particle treatments of thoraco-abdominal tumours): a clinical trial to study the organ motion of thoraco-abdominal neoplasms through 4D MRI;
- INSIDE: an experimental observational real-time live study of the particle range. This study is aimed at the early identification of potential morphological modifications of the target or of the adjacent areas, which might cause an anomaly in the dose distribution.
-
STOPSTORM (a prospective European validation cohort for stereotactic therapy of Re-entrant tachycardia): aimed at the definition and harmonization of ventricular tachycardia radiation therapy treatment options (both medical and ablation therapy); to note that, at the end of 2019, in collaboration with Fondazione IRCSS Polyclinic San Matteo of Pave, for the first time in the literature, a patient affected by ventricular tachycardia (VT) has been successfully treated with proton beams at CNAO [98].
-
PROTECT (PROton versus photon Therapy for Esophageal Cancer—a Trimodality strategy): a randomized clinical study aimed at building scientific evidence (in terms of efficacy and toxicity) on the proton pre-op treatment, combined with chemotherapy, for oesophageal cancer. This clinical trial is then compared to the current gold standard treatment, which is a combination of chemotherapy and IMRT.
- STOPSTORM (a prospective European validation cohort for stereotactic therapy of Re-entrant tachycardia): aimed at the definition and harmonization of ventricular tachycardia radiation therapy treatment options (both medical and ablation therapy); to note that, at the end of 2019, in collaboration with Fondazione IRCSS Polyclinic San Matteo of Pave, for the first time in the literature, a patient affected by ventricular tachycardia (VT) has been successfully treated with proton beams at CNAO [46].
- PROTECT (PROton versus photon Therapy for Esophageal Cancer—a Trimodality strategy): a randomized clinical study aimed at building scientific evidence (in terms of efficacy and toxicity) on the proton pre-op treatment, combined with chemotherapy, for oesophageal cancer. This clinical trial is then compared to the current gold standard treatment, which is a combination of chemotherapy and IMRT.
References
- Haberer, T. Ion Beam Therapy at HIT: Options for Multi-Ion Treatment and Research. In Talk at the 3rd HITRIplus Seminar; Heidelberg University Hospital: Heidelberg, Germany, 13 October 2021; Available online: https://indico.cern.ch/event/1081649/ (accessed on 30 November 2021).
- Krämer, K.; Durante, M. Ion beam transport calculations and treatment plans in particle therapy. Eur. Phys. J. 2010, 60, 195–202.
- Suit, H.; DeLaney, T.; Goldberg, S.; Paganetti, H.; Clasie, B.; Gerweck, L.; Niemierko, A.; Hall, E.; Flanz, J.; Hallman, J.; et al. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother. Oncol. 2010, 95, 3–22.
- Durante, M.; Loeffler, J.S. Charged particles in radiation oncology. Pub. Med. 2009, 7, 37–43.
- Averbeck, N.B.; Topsch, J.; Scholz, M.; Kraft-Weyrather, W.; Durante, M.; Taucher-Scholz, G. Efficient Rejoining of DNA Double-strand breaks despite increased cell-killing effectiveness following spread-out bragg peak carbon-ion irradiation. Front. Oncol. 2016, 6, 1–8.
- Chiblak, S.; Tang, Z.; Campos, B.; Gal, Z.; Unterberg, A.; Debus, J.; Herold-Mendel, C.; Abdollahi, A. Radiosensitivity of patient-derived glioma stem cell 3-dimensional cultures to photon, proton, and carbon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 112–119.
- Peschke, P.; Debus, J. Relative biological effectiveness of carbon ions for local tumor control of a radioresistant prostate carcinoma in the rat. Int. J. Radiat. Oncol. Biol Phys. 2011, 79, 239–246.
- Debus, J.; Abdollahi, A. For the next trick: New discoveries in radiobiology applied to glioblastoma. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e95–e99.
- Klein, C.; Dokic, I.; Mairani, A.; Mein, S.; Brons, S.; Haring, P.; Haberer, T.; Jakel, O.; Zimmerman, A.; Zenke, F.; et al. Overcoming hypoxia-induced tumor radiore-sistance in non-small cell lung cancer by tar-geting DNA-dependent protein kinase in com-bination with carbon ion irradiation. Radiat. Oncol. 2017, 12, 208.
- Takahashi, Y.; Teshima, T.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Madachi, A.; Ikeda, S.; Mizuno, H.; Ogata, T.; Nojima, K.; et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res. 2003, 63, 4253–4257.
- Kamlah, F.; Hänze, J.; Arenz, A.; Seay, U.; Hasan, D.; Juricko, J.; Bischoff, B.; Gottschald, O.R.; Fournier, C.; Taucher-Scholz, G.; et al. Comparison of the effects of carbon ion and photon irradiation on the angiogenic response in human lung adenocarcinoma cells. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1541–1549.
- Ogata, T.; Teshima, T.; Kagawa, K.; Teshima, T.; Kagawa, K.; Hishikawa, Y.; Takahashi, Y.; Kawaguchi, A.; Suzumoto, Y.; Nojima, K.; et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res. 2005, 65, 113–120.
- Rieken, S.; Rieber, J.; Brons, S.; Rieber, J.; Brons, S.; Habermehl, D.; Rief, H.; Orschiedt, L.; Lindel, K.; Weber, K.J.; et al. Radiation-induced motility alterations in medulloblastoma cells. J. Radiat. Res. 2015, 56, 430–436.
- Durante, M.; Brenner, D.J.; Formenti, S.C. Does heavy ion therapy work through the immune system? Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 934–936.
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491.
- Slater, J.M.; Archambeau, J.O.; Dicello, J.F.; Slate, J.D. Proton beam irradiation: Toward routin clinical utilization. In Hadrontherapy in Oncology: Proceedings of the First International Symposium on Hadrontherapy, Como, Italy, 18–21 October 1993; Amaldi, U., Larsson, B., Eds.; Elsevier Science: Amsterdam, The Netherland, 1994; p. 130.
- Kawachi, K.; Yamada, S.; Sato, K.; Ogawa, H.; Soga, F.; Kanai, T.; Endo, M.; Hirao, Y. Heavy ion medical accelerator facility in Japan. In Hadrontherapy in Oncology: Proceedings of the First International Symposium on Hadrontherapy, Como, Italy, 18–21 October 1993; Amaldi, U., Larsson, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; p. 229.
- ENLIGHT. ENLIGHT Coordination. Available online: https://enlight.web.cern.ch/enlight (accessed on 30 November 2021).
- An Organization for Those Interested in Proton, Light Ion and Heavy Charged Particle Radiotherapy. Particle Therapy Co-Operative Group. Available online: www.ptcog.ch (accessed on 30 November 2021).
- Loeffler, J.S.; Durante, M. Charged particle therapy—optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 2013, 10, 411–424.
- Fujita, M.; Yamada, S.; Imai, T. Irradiation induces diverse changes in invasive potential in cancer cell lines. Semin. Cancer Biol. 2015, 35, 45–52.
- Facoetti, A.; Di Gioia, C.; Pasi, F.; Di Liberto, R.; Corbella, F.; Nano, R.; Ciocca, M.; Valvo, F.; Orecchia, R. Morphological analysis of amoeboid-mesenchymal transition plasticity after low and high LET radiation on migrating and invading pancreatic cancer cells. Anticancer Res. 2018, 38, 4585–4591.
- Croce, S.; Peloso, A.; Zoro, T.; Avanzini, M.A.; Cobianchi, L. A Hepatic scaffold from decellularized liver tissue: Food for thought. Biomolecules 2019, 9, 813.
- Zhang, M.; Zhu, Z.-L.; Gao, X.-L.; Wu, J.-S.; Liang, X.-H.; Tang, Y.-L. Functions of chemokines in the perineural invasion of tumors (Review). Int. J. Oncol. 2018, 52, 1369–1379.
- Di Renzo, M.F.; Corso, S. Patient-derived cancer models. Cancers 2020, 12, 3779.
- Friedrich, T.; Henthorn, N.; Durante, M. Modeling radioimmune response-current status and perspectives. Front. Oncol. 2021, 11, 647272.
- Iannalfi, A.; D’Ippolito, E.; Riva, G.; Molinelli, S.; Gandini, S.; Viselner, G.; Fiore, M.R.; Vischioni, B.; Vitolo, V.; Bonora, M.; et al. Proton and carbon ion radiotherapy in skull base chordomas: A prospective study based on a dual particle and a patient-customized treatment strategy. Neurol. Oncol. 2020, 22, 1348–1358.
- Van Dijk, B.A.; Gatta, G.; Capocaccia, R.; Pierannunzio, D.; Strojan, P.; Licitra, L.; The RARECARE Working Group. Rare cancers of the head and neck area in Europe. Eur. J. Cancer 2012, 48, 783–796.
- El-Naggar, A.K.; Chan, J.F.K.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; Volume 9.
- Vischioni, B.; Dhanireddy, B.; Severo, C.; Bonora, M.; Ronchi, S.; Vitolo, V.; Fiore, M.R.; D’Ippolito, E.; Petrucci, R.; Barcellini, A.; et al. Reirradiation of salivary gland tumors with carbon ion radiotherapy at CNAO. Radiother. Oncol. 2020, 145, 172–177.
- Loap, P.; Vischioni, B.; Bonora, M.; Ingargiola, R.; Ronchi, S.; Vitolo, V.; Barcellini, A.; Goanta, L.; De Marzi, L.; Dendale, R.; et al. Biological rationale and clinical evidence of carbon ion radiation therapy for adenoid cystic carcinoma: A narrative review. Front. Oncol. 2021, 11, 789079.
- RARECAREnet. Information Network on Rare Cancers. 2017. Available online: http://www.rarecarenet.eu/rarecarenet/ (accessed on 30 November 2021).
- Kirchoff, D.D.; Deutsch, G.B.; Foshag, L.J.; Lee, J.H.; Sim, M.-S.; Faries, M.B. Evolving therapeutic strategies in mucosal melanoma have notiImproved survival over five decades. Am. Surg. 2016, 82, 1–5.
- Teterycz, P.; Czarnecka, A.M.; Indini, A.; Spałek, M.J.; Labianca, A.; Rogala, P.; Cybulska-Stopa, B.; Quaglino, P.; Ricardi, U.; Badellino, S.; et al. Multimodal treatment of advanced mucosal melanoma in the era of modern immunotherapy. Cancers 2020, 12, 3131.
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Melanoma of the lower genital tract: Prognostic factors and treatment modalities. Gynecol. Oncol. 2018, 150, 180–189.
- Penel, N.; Mallet, Y.; Mirabel, X.; Van, J.T.; Lefebvre, J.-L. Primary mucosal melanoma of head and neck: Prognostic value of clear margins. Laryngoscope 2006, 116, 993–995.
- Shuman, A.G.; Light, E.; Olsen, S.H.; Pynnonen, M.A.; Taylor, J.M.; Johnson, T.M.; Bradford, C.R. Mucosal melanoma of the head and neck: Predictors of prognosis. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 331–337.
- Lee, S.P.; Shimizu, K.T.; Tran, L.M.; Juillard, G.; Calcaterra, T.C. Mucosal melanoma of the head and neck: The impact of local control on survival. Laryngoscope 1994, 104, 121–126.
- Ebner, D.K.; Malouff, T.D.; Frank, S.J.; Koto, M. The role of particle therapy in adenoid cystic carcinoma and mucosal melanoma of the head and neck. Int. J. Part Ther. 2021, 8, 273–284.
- Murata, H.; Okonogi, N.; Wakatsuki, M.; Kato, S.; Kiyohara, H.; Karasawa, K.; Ohno, T.; Nakano, T.; Kamada, T.; Shozu, M.; et al. Long-term outcomes of carbon-ion radiotherapy for malignant gynecological melanoma. Cancers 2019, 11, 482.
- Barcellini, A.; Vitolo, V.; Facoetti, A.; Fossati, P.; Preda, L.; Fiore, M.R.; Vischioni, B.; Iannalfi, A.; Bonora, M.; Ronchi, S.; et al. Feasibility of carbon ion radiotherapy in the treatment of gynecological melanoma. In Vivo 2019, 33, 473–476.
- Takayasu, Y.; Kubo, N.; Shino, M.; Nikkuni, O.; Ida, S.; Musha, A.; Takahashi, K.; Hirato, J.; Shirai, K.; Saitoh, J.; et al. Working Group on Head and Neck Tumors. Carbon-ion radiotherapy combined with chemotherapy for head and neck mucosal melanoma: Prospective observational study. Cancer Med. 2019, 8, 7227–7235.
- Cavalieri, S.; Ronchi, S.; Barcellini, A.; Bonora, M.; Vischioni, B.; Vitolo, V.; Villa, R.; Del Vecchio, M.; Licitra, L.; Orlandi, E. Toxicity of carbon ion radiotherapy and immune checkpoint inhibitors in advanced melanoma. Radiother. Oncol. 2021, 164, 1–5.
- ClinicalTrials.gov. The U.S. National Library of Medicine. ID: NCT03822936. Available online: https://ClinicalTrials.gov (accessed on 30 November 2021).
- Vitolo, V.; Cobianchi, L.; Brugnatelli, S.; Barcellini, A.; Peloso, A.; Facoetti, A.; Vanoli, A.; Delfanti, S.; Preda, L.; Molinelli, S.; et al. Preoperative chemotherapy and carbon ions therapy for treatment of resectable and borderline resectable pancreatic adenocarcinoma: A prospective, phase II, multicentre, single-arm study. BMC Cancer 2019, 19, 922.
- Dusi, V.; Vitolo, V.; Frigerio, L.; Totaro, R.; Valentini, A.; Barcellini, A.; Mirandola, A.; Perego, G.B.; Coccia, M.; Greco, A.; et al. First-in-man case of non-invasive proton radiotherapy for the treatment of refractory ventricular tachycardia in advanced heart failure. Eur. J. Heart Fail. 2020, 23, 195–196.
- Niemierko, A.; Goitein, M. Calculation of normal tissue complication probability and dose-volume histogram reduction schemes for tissues with a critical element architecture. Radiother. Oncol. 1991, 20, 166–176.
- Langendijk, J.A.; Lambin, P.; De Ruysscher, D.; Widder, J.; Bos, M.; Verheij, M. Selection of patients for radiotherapy with protons aiming at reduction of side effects: The model-based approach. Radiother. Oncol. 2013, 107, 267–273.
- Langendijk, J.A.; Hoebers, F.J.P.; de Jong, M.A.; Doornaert, P.; Terhaard, C.H.J.; Steenbakkers, R.J.H.M.; Hamming-Vrieze, O.; van de Kamer, J.B.; Verbakel, W.F.A.R.; Keskin-Cambay, F.; et al. National protocol for model-based selection for proton therapy in head and neck cancer. Int. J. Part Ther. 2021, 8, 354–365.
- Tambas, M.; Steenbakkers, R.J.H.M.; van der Laan, H.P.; Wolters, A.M.; Kierkels, R.G.J.; Scandurra, D.; Korevaar, E.W.; Oldehinkel, E.; van Zon-Meijer, T.W.H.; Both, S.; et al. First experience with model-based selection of head and neck cancer patients for proton therapy. Radiother. Oncol. 2020, 151, 206–213.
- Dionisi, F.; Widesott, L.; Van Vulpen, M.; Fuller, C.D.; Frondizi, R.; Meneguzzo, M.; Blanchard, P.; Amichetti, M.; Sanguineti, G. Methodologies to increase the level of evidence of real-life proton therapy in head and neck tumors. Int. J. Part Ther. 2021, 8, 328–338.
