Osteoporosis is a chronic debilitating disease caused by imbalanced bone remodeling processes that impair the structural integrity of bone. Over the last ten years, the association between fibroblast growth factor 23 (FGF23) and osteoporosis has been studied in both pre-clinical and clinical investigations. FGF23 is a bone-derived endocrine factor that regulates mineral homeostasis via the fibroblast growth factor receptors (FGFRs)/αKlotho complex. These receptors are expressed in kidney and the parathyroid gland. Preclinical studies have supported the link between the local actions of FGF23 on the bone remodeling processes. In addition, clinical evidence regarding the effects of FGF23 on bone mass and fragility fractures suggest potential diagnostic and prognostic applications of FGF23 in clinical contexts, particularly in elderly and patients with chronic kidney disease. However, inconsistent findings exist and there are areas of uncertainty requiring exploration.
- FGF23
- osteoblast
- osteoclast
- bone remodeling
- osteoporosis
1. Introduction
2. Role of FGF23 in Postmenopausal and Age-Related Osteoporosis Pathogenesis
3. Potential Clinical Application of FGF23 in Osteoporosis and Chronic Kidney Disease–Mineral and Bone Disease (CKDD-MBD)
4. FGF23 Measurement in Routine Clinical Practices
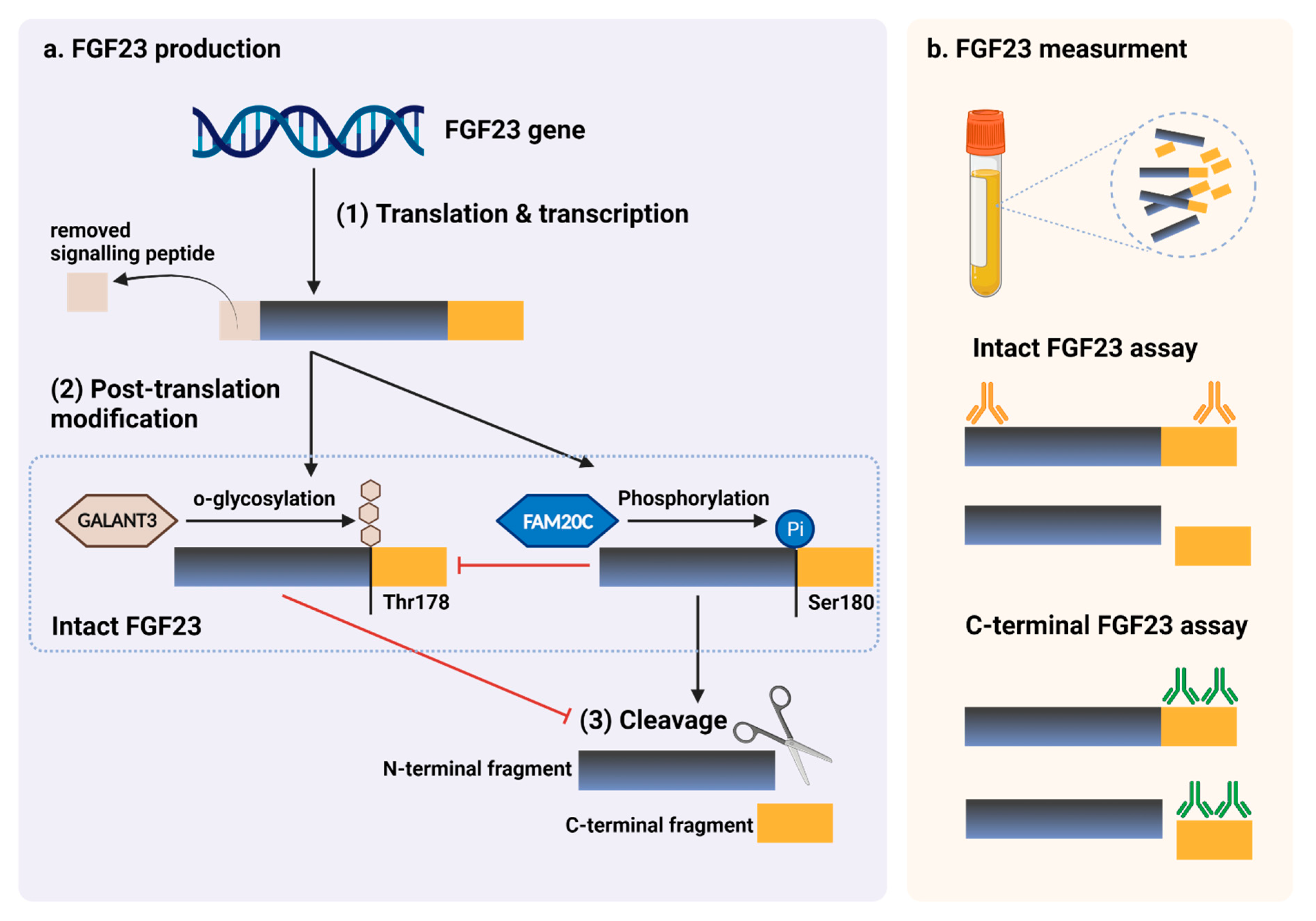 Figure 14. FGF23 production and immunoassay measurements. (a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.
Figure 14. FGF23 production and immunoassay measurements. (a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.