Developmental neurotoxicity (DNT) of chemical compounds disrupts the formation of a normal brain. One of the fundamental features of brain development is the specificity of the anatomical wiring. Neurons elongate their nascent axons by a growth cone positioned at the axon tip. Multiple extracellular guidance cues are integrated by the signal transduction pathways of the growth cone, ultimately resulting in motility changes and goal-directed navigation. To devise an assay for the detection of developmental neurotoxicants targeting correct axonal outgrowth, locust embryos are incubated with a test set of heavy metals, pesticides, and pharmaceuticals that are well recognized as developmental neurotoxic or general cytotoxic compounds. Since axon guidance cues, such as the semaphorin family, are conserved between invertebrates, vertebrates, and mammalians, defects in the navigation of locust pioneer neurons are indicative for developmental neurotoxic effects on the wiring of the mammalian brain.
- axonal pathfinding
- embryo culture
- semaphorin
- directed cell migration
1. Introduction
To devise an assay for the wiring of a functional nere detection of developmental neurotoxicants targeting correct axonal outgrowth, locust embryos are incubated with a test set of heavy metals, pesticides, and pharmaceuticals that are well recognized as developmental neurotoxic or general cytotoxic compounds. Since axon guidance cues, such as the semaphorin family, are conserved between invertebrates, vertebrates, and mammalians, defects in the navigation of locust pioneer neurons are indicative for developmental neurotoxic effects on the wiring of the mammalian brain.
The wiring of a functional nervous system requires precisely timed axonal pathfinding to the correct cellular targets. To address this complex key event, an alternative assay based on serum-free culture of intact locust embryos was developed. The first neural pathways in the leg of embryonic locust are established by a pair of afferent pioneer neurons which use guidance cues from membrane-bound and diffusable semaphorin proteins. In a systematic approach according to recommendations for alternative testing, the embryo assay quantifies defects in pioneer navigation after exposure to a panel of recognized test compounds for DNT. The outcome indicates a high predictability for test compound classification. Since the pyramidal neurons of the mammalian cortex also use a semaphorin gradient for neurite guidance, the assay is based on evolutionary conserved cellular mechanisms supporting its relevance for cortical development.
2. DNT Studies in Invertebrate Model Systems
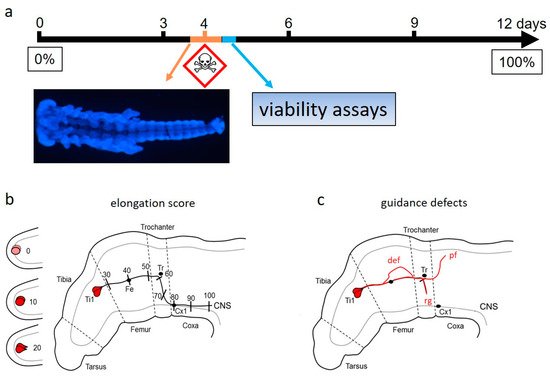
Using intact invertebrates in DNT studies combines the accessability for test substances of in vitro studies with the advantage for providing an in vivo organismal development. The review briefly covers selected examples of DNT studies in the nematode Caenorhabditis elegans and the fly Drosophila melanogaster. Main parts describe in more details a recent approach to design a DNT-assay for correct anatomical wiring, using another classical model system of developmental neurobiology. This approach follows the recommendations for the selection of reference compounds and appropriate use in alternative DNT assays. Intact locust embryos are cultured in serum-free medium to analyze DNT effects of chemical compounds on axonal pathfinding of a pair of identified pioneer neurons. Results from a panel of established DNT-positive and -negative test compounds support a rather high human predictability of this assay [1][2][3][1][2][3].
Established developmental neurotoxicants, such as methyl mercury, arsenic, and the anti-epileptic agent valproic acid, proved DNT-positive in the insect assay. In accordance with its selective inhibitory effects on the axonal outgrowth of human neurons, the mitochondrial respiratory chain blocker rotenone classified as a specific developmental neurotoxicant for pioneer axon elongation and navigation. Apart from blocking the respiratory chain complex I, rotenone also interferes with the cytoskeletal dynamics of microtubule assembly and the RhoA/ROCK pathway. Small molecule blockers of the Rho/ROCK pathway promote neurite extension of cultured human model neurons. The ROCK inhibitor Y27632 partially restored the rotenone-induced decrement of pioneer axon elongation. This rescue experiment supports the classification of rotenone as a specific developmental neurotoxicant.
Establishment of DNT assays require endpoint-specific controls with known mechanistic actions. Growth cone motility driving axonal extension should be affected by blocking cytoskeletal dynamics. The actin polymerization inhibitor cytochalasin D and the microtubule inhibitor colchicine caused on and found a concentration-dependent reduction of axon elongation while general viability did not decrease. Cytoskeletal dynamics in migrating growth cones is highly dependent on alterations in cytosolic calcium levels. , which also applies for pioneer neurons. L-type calcium channel inhibitors reduced pioneer axon elongation in a dose-dependent manner. A comparison with the curves for viability revealed an endpoint-specific DNT-positive classification.
Successful DNT-assessment has to strike a balance between rapidity of performance for testing many chemicals and the accuracy with which the toxicological assays reflect the key developmental processes of the nervous system. The pioneer assay is fast enough to avoid chemical decay, but it allows for the read-out of the essential morphological endpoints in a critical time window. To provide a quantification of axon extension and pathfinding error in concentration–response curves, it weas relied on a rapid scoring scheme based on landmarks [1]. This method is largely independent of the exact limb bud orientation in the focal plane of the fluorescence microscope and a trade-off between the accurate determination of axon length in 3D images and speed of assay quantification.
Scanning laser optical tomography (SLOT) is a novel and relatively fast 3D-imaging technology. The resolution of SLOT was also sufficient to determine the exact anatomical shape of the pioneer neurons in locust embryos [3]. A segmentation algorithm was used to outline the 3D shape of the pioneers. So far, this algorithm has required the manual setting of a fluorescence intensity threshold for the removal of background. A technical advance would be a fully automated image recognition tool for a more rapid and completely unbiased evaluation of the pioneer neuron shape.
Even though the pioneer neuron assay has, so far, only been used to track the formation of a specific axonal pathway, it can be expanded to other endpoints, such as neurogenesis, synaptogenesis, and programmed cell death. Despite the impressive progress in the development of alternative DNT testing methods, this assay seems to be the first procedure that quantifies pathfinding errors of single, identifiable neurons in concentration-response curves.
References:
- Bergmann, G.A.; Froembling, S.; Joseph, N.; Bode, K.; Bicker, G.; Stern, M. An intact insect embryo for developmental neurotoxicity testing of directed axonal elongation. ALTEX 2019, 36, 643–649. https://doi.org/10.14573/altex.1901292.
- Bode, K.; Bohn, M.; Reitmeier, J.; Betker, P.; Stern, M.; Bicker, G. A locust embryo as predictive developmental neurotoxicity testing system for pioneer axon pathway formation. Toxicol. 2020, 94, 4099–4113, doi:10.1007/s00204-020-02929-6.
- Bode, K.; Nolte, L.; Kamin, H.; Desens, M.; Ulmann, A.; Bergmann, G.A.; Betker, P.; Reitmeier, J.; Ripken, T.; Stern, M.; et al. Scanning laser optical tomography resolves developmental neurotoxic effects on pioneer neurons. Rep. 2020, 10, 2641, doi:10.1038/s41598-020-59562-7.
References
- Bergmann, G.A.; Froembling, S.; Joseph, N.; Bode, K.; Bicker, G.; Stern, M. An intact insect embryo for developmental neurotoxicity testing of directed axonal elongation. ALTEX 2019, 36, 643–649. https://doi.org/10.14573/altex.1901292.
- Bode, K.; Bohn, M.; Reitmeier, J.; Betker, P.; Stern, M.; Bicker, G. A locust embryo as predictive developmental neurotoxicity testing system for pioneer axon pathway formation. Toxicol. 2020, 94, 4099–4113, doi:10.1007/s00204-020-02929-6.
- Bode, K.; Nolte, L.; Kamin, H.; Desens, M.; Ulmann, A.; Bergmann, G.A.; Betker, P.; Reitmeier, J.; Ripken, T.; Stern, M.; et al. Scanning laser optical tomography resolves developmental neurotoxic effects on pioneer neurons. Rep. 2020, 10, 2641, doi:10.1038/s41598-020-59562-7.
