Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sergio Granados-Principal and Version 2 by Amina Yu.
Nanomedicine has gained great importance in cancer immunotherapy, as many emerging nanoplatforms with different designs have been recently developed to overcome various mechanisms of cancer immune escape and increase the efficacy of antitumor immune response.
- breast cancer
- nanomedicine
- immune escape
- cancer immunotherapy
- cancer treatment
1. Nanomedicine as a Tool to Overcome Mechanisms of Cancer Immune Escape: A Promising Strategy to Treat Breast Cancer
During the last decade, nanotherapy has been gaining relevance in the immunotherapy field because of its numerous advantages over conventional therapies. The size and adjustable surface properties of nanoparticles (NPs) provide useful features for treatments. For instance, the high biocompatibility and stability of nanocarriers enhance the blood circulation of drugs. Furthermore, nanosystems exhibit a greater accumulation in tumor tissues than traditional treatments since they are sufficiently small to overcome physical and biological barriers and extravasate by passive targeting, which is known as the enhanced permeation and retention (EPR) effect [1][2][3][52,53,54]. In addition, NPs possess remarkable tumor specificity as they are able to accommodate multiple ligands to be delivered into the tumor area by active targeting. Hence, cancer nanomedicine exhibits higher therapeutic efficacy and reduces the systemic toxicity of carried drugs. On the other hand, nanoformulations are able to carry large loads and allow the administration of different cancer drugs in the same application to enhance their antitumor effects. Moreover, NP-based strategies in several mouse breast cancer models were able to convert poorly immunogenic or “cold” tumors into immunogenic or “hot” tumors, establish a T-cell memory immune response, inhibit tumor growth and metastasis and prevent tumor relapse [4][19]. Thus, many novel nanomedicine-based therapies have been proposed to alleviate immunosuppression in tumors and reduce the emergence of tumor escape variants in patients with breast cance.
2. Applications of Nanomedicine to Target Immunosuppressive Tumor Metabolism and Immunosupressive Cytokines in the TME
IL-10 is an immunosuppressive cytokine with an important role in the TME and high levels of serous IL-10 have been associated with poor survival in breast cancer patients. Thus, targeting IL-10 has the potential of becoming an effective therapy for this disease. In this context, lipid-protamine-DNA (LPD) NP platform was loaded with plasmids encoding small antibody-like IL-10 protein trap and locally delivered into a murine 4T1 TNBC model. This IL-10 protein trap caused a regional and transient reduction in IL-10 production and an increased expression of proinflammatory cytokines TNF-α and IFN-γ in the TME. Hence, the IL-10 trap significantly inhibited tumor growth by decreasing M2 macrophages, MDSCs and TFG-β production in the tumor, and enhanced the median survival rate in the in vivo model [5][55].
Growing tumors are also characterized by vascular abnormalities, high lactate levels and glucose deprivation, which lead to acidic pH and hypoxia conditions within the tumor. Both factors have immunosuppressive effects and significantly affect the clinical outcomes in many cancer therapies [6][7][134,135]. For that reason, nanomedicine has also been used to regulate hypoxia and acidity in the TME. Novel designed biodegradable hollow manganese dioxide (H-MnO2) nanoshells were co-loaded with the photodynamic agent chlorine e6 (Ce6) and the chemotherapy drug doxorubicin (Dox), generating a nanoplatform (H-MnO2-PEG/C&D) that is dissociated under reduced pH levels within the TME to specifically release loaded drugs or cargo in the tumor area. Furthermore, this nanoplatform could reduce local tumor hypoxia by inducing decomposition of tumor endogenous H2O2 into water and oxygen and further promote photodynamic therapy (PDT), in which oxygen is actively involved. In this way, after systemic injection of H-MnO2-PEG/C&D into 4T1 tumor-bearing mice and light exposure, tumor-associated Tregs population was reduced, while tumor infiltration of CTLs and TAMs was significantly increased with a notably M1 polarization. More significantly, a combined treatment with this nanoplatform and PD-L1 blockade potentiated antigen-specific CTLs and inhibited the growth of light-irradiated primary tumors cells as well as protecting against light-exposure tumors [8][56]. Similarly, core-shell gold nanocage@manganese dioxide (AuNC@MnO2, AM) NPs have been developed to increase oxygen concentration in the TME, with the aim of enhancing the PDT treatment in a metastatic TNBC murine model. The acidic pH of the TME breaks down the nanoplatform shell and NPs generate a massive release of oxygen in the tumor area. In turn, oxygen-boosted PDT elicits the immunogenic cell death (ICD) of cancer cells (Figure 1), followed by the liberation of damage-associated molecular patterns (DAMPs) and their subsequent presentation by mature DCs to effector immune cells. Therefore, the nanoplatform combined with PDT in this model was able to induce a systemic antitumor immune response, destroy primary tumors and prevent tumor metastases [9][57].
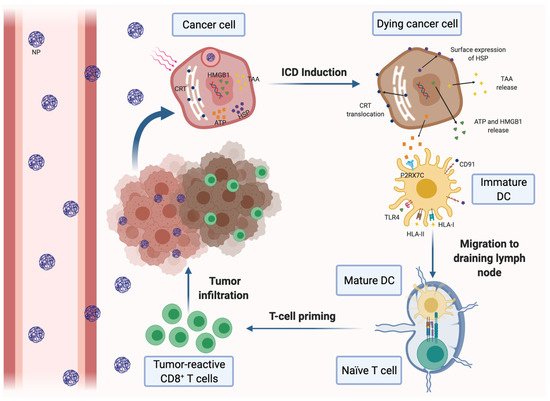
Figure 1. Induction of immunogenic cell death (ICD) of cancer cells by nanomedicine-based photodynamic or photothermal therapy. Nanoparticles (NPs) carrying a photodynamic agent extravasate blood vessels, reach breast tumors and are internalized by cancer cells, where they release their loading. Upon laser irradiation, the dying cancer cells secrete tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs), such as ATP and high-mobility group box 1 protein (HMGB-1). Tumor cells also express DAMPs on the cell surface, including calreticulin (CRT) and heat-shock proteins (HSP). DAMPs can be recognized by different pattern recognition receptors expressed on dendritic cells (DCs), resulting in their maturation and activation. Mature DCs migrate to the draining lymph nodes and induce the activation of tumor-specific effector CD8+ and CD4+ T cells, which infiltrate the tumor and mediate the specific antitumor immune response.
In another approach, small interference RNA (siRNA) cationic lipid-assisted NPs (CLAN) were designed to neutralize tumor acidity by inactivating lactate dehydrogenase A (LDHA). In cancer cells, LDHA converts pyruvate to lactic acid because of elevated tumor aerobic glycolysis, which leads to the pH decreasing in the TME. Tumor acidity promotes CD8+ T cells apoptosis and anergy, while high levels of lactate enhance the function of MDSCs and Tregs and polarize TAMs to the M2 phenotype. siRNA CLAN were systematically injected into a murine 4T1 mammary tumor model to downregulate lactate production and neutralize tumor pH before the administration of an anti-PD-1 antibody. Hence, the efficacy of PD-1 blockade therapy was potentiated because tumor infiltration with CD8+ T and NK cells was increased and their functions were restored, whereas tumor-associated Treg density was reduced [10][58].
3. Applications of Nanomedicine to Target Immunosuppressive Cells within the TME
Nano-sized strategies have also been considered to inhibit the immunosuppressive effects of different immune cells that are abundantly found in the TME in breast cancer.
3.1. Nanomedicine-Based Approaches for Targeting Neutrophils
Recently, cancer vaccination with virus-like particles (VLP), involving the spontaneous and non-infectious organization of viral coat proteins into the structure of a particular virus capsid, has been examined due to the intrinsic immunogenic properties that stimulate the immune response. In this way, an empty cowpea mosaic virus (eCPMV) VLP system was administered by to Lizzote and co-authors to a 4T1 BALB/c syngeneic breast cancer model by inhalation to act as an in-situ vaccine. This system demonstrated an antitumor effect based on the activation of tumor-infiltrating N1 neutrophils, which finally induced a systemic antitumor immunity. N1 neutrophils coordinate the adaptive immune response, produce pro-inflammatory cytokines, recruit T and NK cells and stimulate antitumor cytotoxicity. Upon treatment, both a significant delay of lung metastasis onset and an extended survival of animals were observed [11][136].
3.2. Nanomedicine-Based Approaches for Targeting NK Cells
With the purpose of promote antitumor activity of NK cells in the immunosuppressive TME, treatment with extracellular vesicles (EVs) derived from NK cells was investigated by Zhu et al. In this case, EVs were isolated from human NK cells treated with IL-15 (NK-EVsIL-15) and incubated with different cancer cell lines, including the breast cancer cell line MDA-MB-231/F. The priming of NK cells with IL-15, a key cytokine that promotes NK cell proliferation and survival, enhances their cytotoxicity. It also increases the secretion of EVs and promotes the NK-EVsIL-15 immunotherapeutic effects due to increased production of cytotoxic proteins (perforin and granzyme B), overexpression of the membrane Fas ligand (FasL) and improved tumor-targeting ability, when compared with EVs from untreated NK cells (NK-EVs). Moreover, NK-EVsIL-15 had a higher ability to induce apoptosis in cancer cells than NK-EVs and they were able to inhibit the growth of glioblastoma xenografts in mice [12][59].
3.3. Nanomedicine-Based Approaches for Targeting Macrophages
TAMs have been reported to occupy up to 50% of breast tumors and most of them exhibit the M2 phenotype, and exert immunosuppressive and tumor-promoting effects. Thus, an important approach to battle cancer is to repolarize TAMs to the M1 phenotype, with immunostimulatory and tumor-inhibiting properties. Several strategies are currently being evaluated in order to achieve this goal in breast tumors. One of them consists in the systemic delivery of dual-pH responsive NPs that carry siRNAs targeting VEGF and placental growth factor (PIGF). Under acidic TME, both siRNAs were released and internalized into TAMs and breast cancer cells by mannose-mediated phagocytosis and passive targeting, respectively. These NPs downregulated VEGF and PIGF expression in cancer cells and M2-like TAMs, repolarized M2-TAMs to the M1 phenotype, reduced macrophage recruitment, angiogenesis and hypoxia within the TME and slowed down breast tumor growth and lung metastasis in the treated mice [13][137].
TAMs are polarized to the M2 phenotype upon the interaction between its colony-stimulating factor 1 receptor (CSF1R) and the macrophage colony-stimulating factor 1 (MCSF) produced by cancer cells. The binding of MCFS to CSF1R results in the activation of multiple downstream signaling pathways, including the mitogen-activated protein kinase (MAPK) pathway, which stimulates the proliferation and survival of M2-like TAMs. Therefore, another promising strategy to repolarize M2-like TAMs to the M1 phenotype could be based on the therapeutic vertical inhibition of the CSF1R and MAPK signaling pathways. Dual-kinase inhibitor-loaded supramolecular NPs that are able to co-deliver CSF1R and MAPK inhibitors into TAMs were used in an aggressive 4T1 breast cancer model and they enhanced the efficiency of M2-to-M1 repolarization, as well as anti-tumor efficacy [14][60]. Moreover, iron oxide NPs (ferumoxytol) were also demonstrated to induce M2-to-M1 repolarization of TAMs in the TME. These NPs were internalized by TAMs and increased the mRNA associated with Th1-type response, while the presence of M1 in the TME was augmented. Thus, antitumor cytotoxicity was improved and the growth of subcutaneous adenocarcinomas was inhibited in mice [15][61].
Another approach is based on the use of small molecule-gold nanorod conjugates to selectively target and induce macrophage cytotoxicity towards breast cancer cells in vitro. Gold NPs have been demonstrated to serve as targeted drug delivery vehicles and to act as contrast agents for near-infrared (NIR) laser photothermal tumor ablation. For that reason, colloidal gold nanorods (AuNRs) were functionalized with macrolides, which are antibiotics that are targeted towards macrophages. Since solid tumors are highly infiltrated with macrophages, this approach may facilitate the preferential delivery of the nanoconjugates to the tumor site. In this experimental model, AuNRs were preferentially delivered into TAMs, induced their antitumor activity and cytotoxicity against breast adenocarcinoma cells in co-culture and increased the efficacy of laser photothermal therapy (PTT). In addition, cytotoxic TNF-α and IL-1/6 protein levels were increased in the AuNR-treated macrophages [16][62].
3.4. Nanomedicine-Based Approaches for Targeting CAFs
CAFs are also key stromal cells responsible for the formation of the immunosuppressive TME in solid tumors because they can recruit immunosuppressive cells and produce a variety of growth factors that promote tumor growth. Furthermore, they create a physical barrier for both drug delivery and tumor infiltration with cytotoxic T cells. To overcome immune evasion mediated by these cells, depletion of this lymphocyte population was considered as a therapeutic possibility. However, according to a recent report, their elimination caused the upregulation of Wnt16, which rendered the neighboring tumor cells resistant to treatment [17][138]. Hence, the inhibition of tumor CAFs seems to be a more attractive approach. In this context, a novel puerarin nanoemulsion (nanoPue) was developed to downregulate reactive oxygen species (ROS) production in activated CAFs. ROS are actively involved in multiple profibrogenic pathways and are indispensable for CAFs’ activation, and thus, nanoPue demonstrated a strong ability to inactivate CAFs in the TME. As a consequence, collagen deposition in the tumor area was reduced and tumor permeability was enhanced, which improved the chemotherapy effect in the desmoplastic TNBC model, induced a two-fold increase in tumor infiltration with cytotoxic T cells and decreased tumor weight. Moreover, remodeling of TME improved the efficacy of PD-L1 blockade therapy in a TNBC model. Therefore, nanoPue could serve as an adjuvant therapy for chemotherapeutic drugs and immunotherapies in highly desmoplastic tumors, such as TNBC [18][63].
One different strategy to inhibit CAFs in the TME consists in the injecting a hydrogel to controllably deliver losartan into the tumor area. TGF-β is a key driver of CAFs’ activity as it induces the secretion of collagen I by CAFs, which can stabilize the epithelial-to-mesenchymal transition and promote the invasion of cancer cells and tumor metastasis. Losartan is an angiotensin II receptor antagonist, which inhibits the TGF-β signaling pathway and inactivates CAFs after prolonged and localized administration. Thus, losartan was trapped within a peptide hydrogel network and intratumorally injected in a TNBC murine model. Notably, these polymeric hydrogels have a great potential because they can be easily functionalized, delivered by local injection and degraded into amino acids afterwards. Upon the injection of the hydrogel, Dox-loaded liposomes (Dox-L) were also administered. Losartan hydrogel improved the efficacy of Dox chemotherapy using CAF inactivation, leading to more effective inhibition of tumor growth (64% in comparison with Dox-L alone) and lung metastasis (80% versus Dox-L alone). The described data suggest the high therapeutic potential of this adjuvant therapy [19][64].
3.5. Nanomedicine-Based Approaches for Targeting MDSCs
MDSCs are highly accumulated in tumor sites, where they are able to suppress the activation and proliferation of cytotoxic T cells and stimulate Tregs cells. Hence, nanomedicine-mediated depletion of MDSCs in the TME could be a new perspective in cancer immunotherapy. Examples include a syringeable immunomodulatory multidomain nanogel (iGel), which was designed to both reduce MDSCs in tumors and reprogram the whole TME from a tumor-promoting to a tumor-rejecting immune niche. A positive therapeutic effect of the intratumoral depletion of inhibitory TAMs and MDSC was described in a 4T1 murine model after the application of iGel. This synthetic immune niche was applied as a local postsurgical treatment in the 4T1 murine model to achieve a local and extended release of clodronate, in order to deplete inhibitory TAMs, together with gemcitabine (GEM) and the Toll-like receptor 7 (TLR7) agonist imiquimod (R837), with the aim of triggering the ICD of tumor cells, activating recruited APCs, generating antigen-specific T cells and depleting inhibitory MDSCs at the tumor site. After the treatment with iGel, the TME was characterized by increased levels of infiltrating CD4+ and CD8+ T cells and NK cells, upregulated expression of Th1 cytokines and reduced percentages of MDSCs and M2-like TAMs. In addition, iGel was able to generate a memory T-cell response and inhibit tumor growth and lung metastasis. Finally, the expression levels of PD-1 in T cells and PD-L1 in tumor cells were higher in treated 4T1 tumor-bearing mice. Thus, tumors initially not responding to checkpoint therapy became sensitive to this treatment [20][65].
A similar implantable synthetic immune niche (iCD) was designed using the MSDCs-depleting agent GEM and a cancer vaccine, composed of whole-tumor lysate antigens together with TLR3 agonists to promote a stronger immune response. The interconnected porous architecture of the iCD provides both local and sustained delivery of drugs and a space for recruited immune cells to interact with nanomedicines and become activated. The iCD acts as an immune-priming center that induces the recruitment and activation of DCs and the proliferation of antigen-specific T cells, reduces the population of MDSCs in the TME and creates a favorable microenvironment for T-cell activity via the local delivery of GEM. Moreover, iCD was successfully used as a postsurgical tumor treatment in an advanced-stage primary 4T1 breast tumor model. It was reported to prevent tumor recurrence at the surgical site, reduce lung metastasis in 60% of the treated mice and achieve a 100% survival rate [21][66].
In addition, treatment with Dox was suggested to selectively eliminate MDSCs in the spleen and TME and was shown to have immunomodulatory effects [22][139]. Thus, this chemotherapeutic agent has been proposed to be used as a part of nanomedicines in different types of malignancy, including breast cancer. One of these strategies is a combined administration of a liposomal nanoformulation of HER2/neu-derived P5 peptide together with PEGylated liposomal Dox (Doxil®) to treat HER2+ tumor-bearing mice, aiming to inhibit the MDSCs via Doxil® and induce a stronger immune response upon the liposomal P5 immunotherapy. This combination reduced the population and activity of MDSCs in the spleen and TME, while it enhanced the CD4+ and CD8+ T cells’ populations and IFN-γ production. Finally, it decreased tumor size and increased survival rates. Importantly, Doxil® is less cardiotoxic than free Dox and more efficient in MDSC elimination and CTL activation [23][67].
Cancer treatment with the Dox-polyglycerol-nanodiamond conjugate (Nano-DOX) has been also studied. Nano-DOX displays a lower therapeutic potency than free Dox but exhibits some benefits over the free drug. This nanodiamond was used to treat 4T1 breast tumor-bearing mice and showed better tolerance and less toxicity than free Dox, without causing chemoresistance to Dox in the 4T1 cells, which is a key problem of the free chemotherapeutic agent. In addition, nano-DOX downregulated tumor-derived granulocyte-colony-stimulating factor (G-CSF) and suppressed tumor infiltration with MDSCs and MDSCs’ activation. Finally, it induced the release of DAMPs by 4T1 cells and the subsequent activation of M1 macrophages, DCs and CD4+ and CD8+ T cells in the tumor tissue and stimulation of the antitumor immune response. All these findings together demonstrate that chemotherapeutic drugs in nano-forms acquire new improved properties and these nanomedicines in combination with immunotherapy may provide a more effective treatment of cancer [24][68].
3.6. Nanomedicine-Based Approaches for Targeting Tregs
High-grade tumor infiltration with Tregs is correlated with poor prognosis in breast cancer patients as these lymphocytes inhibit CTLs. Hence, depletion of tumor Tregs is another candidate strategy to potentiate cancer immunotherapy. Ursolic acid (UA)-liposomes have been developed to reduce Tregs within the TME in 4T1 tumor-bearing mice. UA is a potent pentacyclic triterpenoid, found naturally in plants and fruits, which shows immunomodulatory effects due to the inhibition of the JAK/STAT signaling pathways in Tregs. Tregs express high levels of the IL-2 receptor α chain (CD25) that binds to IL-2 and causes the phosphorylation of STAT5, the activation of the JAK/STAT signaling pathway and the subsequent induction of Foxp3 transcription. After the injection of UA-liposomes in a murine breast cancer model, they reached the tumor tissue and reduced IL-10 and IL-6 secretion as well as Foxp3+ phenotypic Tregs cells by inhibiting STAT5 phosphorylation. Despite the fact that UA-liposomes are only able to slow down tumor growth without completely destroying tumor tissue, an in vivo administration of these liposomes led to a depletion of MDSCs and CD4+CD25+Foxp3+ Tregs in the TME [25][69]. Systemically administered iron-oxide nanoparticles (IONPs) together with PTT also effectively depleted tumor-associated Tregs in a murine 4T1 breast tumor model. Moreover, IONPs-mediated PTT combined with immune checkpoint blockade can significantly inhibit 4T1 primary and distal metastatic tumor growth through the abscopal effect. Remarkably, the combined therapy was able to generate functional memory tumor-antigen-specific T cells to prevent tumor recurrence [26][70]. ZIn another study, zoledronic acid containing-NPs (NZ) were used to reverse Dox chemoresistance in breast cancer models. NZ inhibited the STAT3/IDO axis and the production of kynurenine, an immunosuppressive catabolite of tryptophan that is produced by IDO. Kynurenine, which is highly expressed on chemoresistant cancer cells, increased the Tregs population and impaired the proliferation and survival of T lymphocytes in the TME. In this study, NZ decreased the number of Tregs and increased the recruitment of DCs in the tumor area, thereby restoring the recognition of resistant tumors via the host immune system. This finding demonstrated that NZ could be used as an adjuvant agent to improve chemo-immunotherapy protocols for chemoresistant breast tumors [27][71].
Lastly, the novel CAR-T (Chimeric Antigen Receptor T)-cell-mediated cancer therapy is very promising, but its therapeutic effect is poor in solid tumors due to the immunosuppressive TME. To overcome this limitation, lipid NPs were designed to enhance CAR-T-cell therapy in breast cancer. These NPs carried a drug cocktail to simultaneously inhibit immunosuppressive cells within the TME and stimulate antitumor immune cells and they were tested in 4T1 tumor-bearing mice before the infusion of CAR-T cells. In particular, these NPs were loaded with an immunostimulant-invariant natural killer T-cell (iNKT) agonist and a selective inhibitor of the phosphoinositide 3-kinase (PI3K) p110δ isoform, which shows activity against tumor-associated Tregs and MDSCs [28][72]. Since p110δ is a relevant signaling protein that controls many functions of immune cells’ physiology, the inhibition of PI3K reduces Tregs’ and MDSCs’ proliferation and activity [29][140]. In this way, prior NPs conditioning reversed the immune-hostile TME and thereby infused CAR-T cells were able to effectively infiltrate the tumor area, undergo robust expansion and eliminate cancer cells. Indeed, preconditioning with these NPs reduced the concentration of TAMs (by 9.4-fold), MDSCs (by 4.6-fold) and Tregs (by 4.8-fold) and increased the density of CD8+ T cells (by 6.2-fold) and iNKT cells (by 29.8-fold) at the tumor site. As a result, NP preconditioning allowed a dramatic accumulation of CAR-T cells in breast tumors (22-fold higher levels) and increased their therapeutic activity. Therefore, this therapy doubled the overall survival when compared to CAR-T-cell therapy alone [28][72].
4. Applications of Nanomedicine to Enhance DC Antigen Presentation and Activity
The role of DCs in the antitumor immune response can be directly boosted by two main nanotherapeutic strategies: the induction of the ICD of cancer cells and the subsequent release of intrinsic TAA into the TME or the administration of exogenous TAA into the patients using nanovaccines as vehicles.
4.1. Nanotherapies for Inducting the ICD of Cancer Cells
Antigen presentation and DC function are often impaired in the immunosuppressive TME. A promising approach to solve both drawbacks is to increase tumor immunogenicity through the induction of the ICD of malignant cells. ICD stimulates antitumor immunity by different effects on cancer cells, including the promotion of surface expression of calreticulin (CRT) and high-mobility group box 1 (HMGB1) in tumor cells and the increasing of adenosine triphosphate (ATP) secretion. On the one hand, CRT is an ‘eat me’ signal that triggers the phagocytosis of dying tumor cells by DCs, whereas HMGB1 binds to TLR4 and promotes DC maturation and tumor antigen presentation to CTLs. On the other hand, ATP is able to stimulate tumor infiltration with CTLs (Figure 1). Thus, several nanotherapeutic strategies have already been described to elicit ICD in breast tumors. Most of them are based on the application of PDT or PTT to the tumors. For instance, biocompatible Zn-pyrophosphate (ZnP) shell NPs loaded with pyrolipid photosensitizer (PS) in the core can be used to treat 4T1 and TUBO bilateral syngeneic mouse models by PDT [30][73]. Similarly, a tumor-targeted polypyrrole NP carrying the camptothecin (CPT) chemotherapeutic agent and a near-infrared dye was used to perform synergistic chemotherapy and PTT in 4T1 tumor-bearing mice [31][74]. Moreover, a polydopamine nanomedicine was simultaneously loaded with carbon dots, a fluorescent agent and the ROS-responsive TLR7/8 agonist resiquimod (R848) to treat 4T1 tumor-bearing mice [32][75]. These nanosystems were able to induce the ICD of cancer cells and were also combined with PD-L1 blockade therapy. As a result, all of the combined therapies elicited a systemic tumor-specific CTL response. Thereby, they potentiated the effects of PD-L1 inhibition, eradicated both light-irradiated and distant tumors and prevented tumor recurrence and metastasis in mouse models. Notably, combined therapies induced stronger immune response and exhibited higher therapeutic efficacy than immunotherapy alone. In conclusion, nanomedicine-based PDT and PTT can be an effective solution to increase the proportion of cancers responding to immunotherapy [30][31][32][73,74,75].
In addition, several cytotoxic drugs, including oxaliplatin (OXA) and Dox, which have been reported to trigger the ICD of cancer cells, have been encapsulated into nanoplatforms to treat breast cancer. On the one hand, an OXA prodrug and a PEGylated PS have been integrated into a TME-activatable vesicle for treatment in a mouse 4T1 breast tumor model. These vesicles are stable in blood circulation until they arrive at the tumor site, where matrix metalloproteinase-2 (MMP-2) cleaves their PEG corona and the acidic TME triggers the surface charge reversal of these vesicles. It enables tumor-specific penetration, the cellular uptake of vesicles and prodrug activation in tumors. This is followed by laser illumination and the nanoplatform induces ROS generation and drug release, which promotes the ICD of cancer cells. Moreover, αCD47 can be intratumorally injected in mice after the delivery of the vesicles and laser irradiation to block CD47, which is a ‘don’t eat me’ signal overexpressed on the surface of tumor cells that prevent their phagocytosis by DCs. In this way, cancer cells can be phagocytosed after treatment and antitumor immunity is further promoted. Hence, the combination of the nanoplatform with CD47 blockade suppressed tumor growth and distant metastasis and prevented tumor recurrence by triggering a long-term antitumor immunity [33][76]. In this context, CD47 inhibition could also be improved by a different strategy based on nanomedicine. An acidity-responsive nanocarrier (NP-siCD47/CCL25) was developed to sequentially release siCD47 into cancer cells and CCL25 protein within tumor stroma. CCL25 is a chemokine and the only ligand for CCR9+ T cells. These cells possess an enhanced potential to be activated and produce proinflammatory cytokines and may mediate stronger antitumor response, as they are able to promote CD8+ T cells’ expansion and survival and to inhibit CD4+ T-cells’ differentiation into Tregs. However, CCL25 is not expressed on TNBC cells; therefore, it was proposed to intratumorally deliver CCL25 into a murine TNBC model in order to increase CCR9+CD8+ T-cell infiltration in the tumor. As a result, the promotion of CCR9+CD8+ T-cell infiltration enhanced the antitumor effect induced through the downregulation of CD47 expression and caused tumor growth arrest and the inhibition of metastatic dissemination [34][77].
In another attempt to induce the ICD of cancer cells in orthotopic 4T1 breast cancer, Dox was encapsulated into highly integrated mesoporous silica NPs (DOX@HIMSNs). DOX@HIMSNs were integrated with a pH and glutathione (GSH) dual-stimulated rotaxane in order to achieve intratumorally specific drug release and were prepared with active targeting and magnetic resonance/computed tomography imaging abilities to increase tumor-positive contrast and guide in vivo therapies. This therapy was able to inhibit tumor growth and metastasis in treated mice [35][78].
Furthermore, versatile cancer cell membrane (CCM)-coated calcium carbonate NPs carrying low-dose Dox and Ce6 (MC/Dox/Ce6) were developed to release in situ TAAs and serve as a DC vaccine for breast cancer treatment. The CCM is capable of targeting the tumor tissue after being administrated into 4T1 tumor-bearing mice, as it is derived from exogenous cancer cells and exhibits tumor-specific proteins on the surface. Upon laser irradiation, ROS are intracellularly generated and DCs are recruited into the TME. In turn, cancer cells undergo ICD and release TAAs to the TEM, which triggers the maturation of DCs in the tumor area. Hence, MC/Dox/Ce6 promoted a strong antitumor immune response and inhibited both primary and distant tumors after a single administration in combination with PDT. In conclusion, MC/Dox/Ce6 integrates the benefits of chemotherapy, immunotherapy and PDT [36][80].
Numerous approaches to promote the ICD of cancer cells using different immunoadjuvants have also been designed. Firstly, the polymeric cooper chelator RPTDH was used to assemble pH-sensitive NPs carrying R848 in order to combine immune activation with the antiangiogenesis effect of RPTDH-induced copper deficiency. Since cooper salts (the levels of which are often elevated in breast cancer cells) are a key element in the disease, this nanoformulation dramatically suppressed tumor angiogenesis, proliferation and motility caused by copper inhibition. Furthermore, R848 induced DC maturation and immune activation. Hence, tumor growth and lung metastasis were noticeably inhibited in treated mice [37][88]. Secondly, NPs characterized by a superior photothermal conversion efficacy (CPCI-NPs) were evaluated as a strategy to carry a PS agent and controllably release R837 adjuvant (CPCI-R837-NPs) into the TME. Synergistic photothermal/immuno-therapy using CPCI-R837-NPs was proven to be considerably effective against breast cancer in a murine model, especially when it was combined with anti-PD-1 antibody treatment. Similarly to the previous results, this therapy was able to eradicate the light-irradiated tumors as well as the light-unexposed ones, through the activation of a systemic antitumor immune response [36][80]. Moreover, a photothermal agent and R837 were co-encapsulated into poly(lactic-co-glycolic) acid (PLGA) NPs. The formed NPs can be used to apply PTT, induce the ICD of cancer cells and generate TAAs, as it exerts vaccine-like functions together with the immunostimulating effects of R837. These NPs, along with an anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibody, induced a strong antitumor memory immune response in 4T1 breast tumor-bearing mice and were able to eliminate primary and secondary tumors and prevent tumor relapses [38][81].
Likewise, Dox and R837 were separately encapsulated in low-molecular-weight heparin (LMWH)-d-α-tocopheryl succinate (TOS) micelles (LT). The two types of LT exerted synergistic anti-tumor effects: LT-Dox induced the ICD of cancer cells, while LT-R837 triggered the secretion of different cytokines and activated macrophages, DCs and Th1 immune response in an orthotopic 4T1 breast cancer. In turn, LMWH and TOS micelles were not only drug carriers, but also exhibited strong anti-metastasis effect because they inhibit different phases of the metastatic spread. However, the consequent increase in the cytokine secretion upregulates the expression of immune checkpoints such as PD-L1; thus, the micelles were administered in combination with a PD-L1 inhibitor to eliminate their negative effects. This multifunctional strategy upregulated the maturation of DCs, increased the secretion levels of TNF-α and IFN-γ, promoted the Th1 immune response, and increased tumor T-cell infiltration with proper CD8+ CTLs/Tregs and effector CD4+ T cells/Tregs ratios. As a result, the proposed therapy significantly reduced tumor volume and inhibited tumor growth and pulmonary metastasis [39][82].
Nanotechnological treatment with Dox and R848, which can promote the maturation and activity of DCs, has been evaluated as well. For example, immune nanoconverters (iNCVs) were encapsulated with R848 and Dox and loaded into a designed scaffold. Dox induces the ICD of cancer cells and acts as an in-situ cancer vaccine, while R848 exhibits immunostimulatory effects and can also transform the MDSCs into tumoricidal APCs and repolarizes TAMs to the M1 phenotype. This nanoplatform promotes immunogenic phenotypes in tumors, enhances systemic and long-term antitumor immune response, transforms α-PD-L1/α-PD-1-nonresponsive tumors into responsive tumors and prevents tumor recurrence and metastasis in a 4T1 breast cancer model [40][83]. In addition, a dual pH-responsive NP system was designed by the encapsulation of R848 with poly(L-histidine) (PHIS) to form nanocores, which were coated with prodrug hyaluronic acid (HA)-Dox. HA-Dox/PHIS/R848 NPs specifically released Dox and R848 into the acidic TME of 4T1 tumor-bearing mice and dramatically inhibited the tumor growth by potentiating the antitumor immune response [41][84].
Oligodeoxynucleotides containing cytosine-guanine motifs (CpG-ODN) are TLR9 agonists that have been evaluated for their application in cancer nanotherapy as immunoadjuvants. NPs composed of a polymeric core and a gold NP-based coat were used to deliver oligodeoxynucleotides containing cytosine-guanine motifs (CpG-ODN), TLR9 agonists, together with zinc phthalocyanine (ZnPc) PS into mouse 4T1 breast cancer cells. Notably, the CpG-ODN were uptaken into TLR9-rich endosomes of plasmacytoid DCs and they specifically activated TLR9 signaling and induced the activation of DCs. Preliminary results demonstrated the benefits of combining the phototoxic and immune-stimulating activities of PDT with the enhancement of DC activation by CpG-ODN to deal with breast cancer [42][86]. Furthermore, CpG-ODN were loaded into light-responsive chitosan-coated hollow CuS NPs and these NPs were combined with PTT in a mouse breast cancer model. The combined therapy has been proved to efficiently induce the ICD of malignant cells and potentiate the immune response against cancer cells. It reduced primary and distant tumor growth more significantly than PTT or immunotherapy therapy alone [43][87].
Lastly, another described strategy to induce the ICD of breast cancer cells by nanotherapy is based on a tumor-specific enhanced oxidative stress polymer conjugate (TSEOP). Under high concentrations of H2O2 and low levels of pH in the TME, TSEOP specifically releases quinone methide (QM) and generates cinnamaldehyde (CA). QM acts as a GSH scavenger, while CA is a ROS amplifier. In this way, through the cooperative depletion of GSH and the generation of ROS, TSEOP is able to induce strong oxidative stress in tumor cells. It subsequently leads to endoplasmic reticulum stress responses and the ICD of cancer cells is induced. TSEOP, characterized by great tumor selectivity, potentiated the maturation of DCs, boosted antitumor-specific immunity and completely eradicated breast tumors in treated mice [44][85].
4.2. Peptide-Based Nanovaccines
The administration of cancer vaccines is an alternative approach to enhance tumor antigens’ presentation to APCs. Peptide-based vaccines are the most common vaccines that have been designed for breast cancer. They enable DCs to present TAAs T cells and elicit antigen-specific immune responses. Several promising peptide vaccines are under ongoing clinical trials in patients with breast cancer, but still with limited efficacy. For that reason, nanocarriers are beginning to be used as cancer vaccines, as they are able to specifically deliver various antigens with improved stability and act as potent immunoadjuvants. Currently, different types of peptide-based nanovaccines are being evaluated for breast cancer therapy, such as VLP-based, liposomal or polymeric vaccines. Additionally, the repertoire of TAA administered in nanovaccines for breast cancer is constantly expanding.
VLPs have been proposed by many studies as immunogenic adjuvants to enhance the potency of peptide vaccines, since their viral RNA is a natural ligand for TLR7 and their protein structures are also immunogenic. A phase I clinical trial, which offers promising preliminary results, was developed to use an anti-HER2/neu vaccine in patients with metastatic breast cancer (MBC). HER2/neu is a protein that is overexpressed in 15–20% of breast cancers, and is thought to promote metastasis and disease progression. Aiming to increase the antigenicity of the self-antigens, three B-cell epitopes derived from the extracellular domain of the HER2/neu protein were coupled to immune-potentiating reconstituted influenza virosomes (IRIV). After three intramuscularly applications of the virosomal formulated vaccine, it elicited specific B- and T-cell immune responses in 8 of 10 patients. In these eight patients, peptide-specific antibodies for all the epitopes were generated and the production of IL-2, IFN-γ and TNF-α was increased, whereas the number of CD4+CD25+Foxp3+Tregs was reduced [45][89].
Influenza VLPs have been also used to deliver HER2 in a glycosylphosphatidylinositol (GPI)-anchored form into breast tumor-bearing mice. After vaccination, strong Th1- and Th2-type immune responses were promoted and the HER2-specific IgG production was enhanced, while the soluble form of GPI-HER2 weakly induced the Th2 response. Furthermore, vaccinated mice were protected against challenge with HER2-expressing tumor [46][90]. Moreover, VLPs with a high-density surface display of the HER2 extracellular domain were demonstrated to stimulate therapeutically potent and durable anti-HER2 CTL-based responses in a murine model. As a result, these VLPs prolonged the survival of treated mice and prevented tumor growth as well as spontaneous tumor development [47][98]. Additionally, a heterologous prime-boost strategy based on the presentation of the unique HER2 B-cell epitope (CH401) by three different VLPs was evaluated to treat HER2+ breast cancer. The three VLPs were based on CPMV, cowpea chlorotic mottle virus (CCMV) and Sesbania mosaic virus (SeMV). Each nanovaccine was sequentially administered in vivo only once in order to focus the immune responses on the epitopes. This regimen of vaccination elicited higher titers of CH401-specific immunoglobulins, stronger Th1-predominant response and more cytotoxicity towards cancer cells than a repeated vaccination regimen. The heterologous prime-boost regimen reduced tumor growth and enhanced survival in treated mice more significantly than traditional vaccination, which proves that novel vaccination strategies could improve their efficacy against cancer [48][99].
Finally, VPLs were proposed as an approach to inhibit cancer stem cells (CSC). CSC are related to tumor relapse, metastasis and therapeutic resistance and they are dramatically abundant in aggressive forms of breast cancer. With this purpose, a VPL-based vaccine (AX08-0M6) was designed using a family of RNA bacteriophages to display on its surface an extracellular domain of human cystine-glutamate antiporter protein xCT, a key element in CSC function that is highly expressed in breast tumors. AX08-0M6 was proven to effectively impact CSC biology via the production of high levels of anti-xCT IgG2. Thus, it inhibited tumor growth and pulmonary metastases in preclinical breast cancer models [49][100].
In addition, liposomal NPs carrying different peptides have also been reported to potentiate the efficacy of vaccines for breast cancer. For instance, multi-epitope P5 peptide was encapsulated into nanoliposomes composed of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP), which stimulates DCs, cholesterol (Chol) and Polyriboinosinic: polyribocytidylic acid (Poly (I:C)), which is a strong immunoadjuvant that enhances Th1 and CTL responses. The formulation was administered to TUBO tumor-bearing mice three times at two-week intervals. Nanoliposomes carrying P5 were introduced into APCs’ cytosol due to their cationic liposomal composition. As a result, this nanoformulation enhanced the release of IFN-γ by CD8+ T lymphocytes, reduced the tumor growth rate and provided protection against tumor regression in treated mice [50][101]. In the same regard, dioleoyl-phosphatidyl ethanolamine (DOPE) was used to design different liposomes carrying P5 in order to release the peptide into the cytosol of APCs, especially in DCs. These liposomes also delivered monophosphoryl lipid A (MPL), which stimulates TLR4 and induces the production of co-stimulatory molecules and inflammatory cytokines via DCs. As a result, the presentation of P5 to CD8+ lymphocytes through APCs was enhanced. The Lip-DOPE-P5-MPL formulation was subcutaneously injected three times into a TUBO tumor mice model and it induced significant CTL response against the P5 antigen and increased IFN-γ secretion by CD8+ T cells. Lip-DOPE-P5-MPL vaccination inhibited tumor growth and extended survival time in treated mice, as the combination of MPL and DOPE has synergistic effects on boosting vaccine efficacy [51][102]. Similarly, the same DOPE liposomal formulation containing MPL and Pan HLA-DR peptide (PADRE) was used to deliver P5 peptide [52][103] or long multi-epitope HER2/neu-derived E75-AE36 (linkage of two short peptides) [53][104] into TUBO-bearing mice. The addition of PADRE to liposomes activated a powerful antitumor response based on CD4+ T helper cells and remarkably enhanced immune responses against P5 and E75-AE36 peptides, when compared to the free peptides or the same liposomal formulations without PADRE [52][53][103,104]. In addition, synthetic long peptides are able to induce stronger long-lived immune responses than short peptides because the synthetic long peptide contains optimum CTLs and Th epitopes. E75 binds to HLA-A2 and HLA-A3 molecules and stimulates CTLs, while AE36 binds to MHC class II molecules and potentiates CD4+ Th and CD8+ T cells [52][53][103,104].
Short E75 and AE36 peptides encapsulated into different nanoliposomes have also been used in combination with other adjuvants. The AE36 peptide was incorporated into nanoliposomes composed of DOTAP, DOPE and cholesterol (DDC) or only DD, together with the CpG motif. After the injection of these liposomal nanoformulations into a TUBO breast tumor murine model, increased IL-4 and IFN-γ secretion, reduced tumor size and prolonged survival time in therapeutic and prophylactic models were observed [54][105]. E75 was incorporated into liposomes composed of phospholipids, cholesterol and DOPE and similarly promising results were obtained in TUBO tumor-bearing mice [55][91]. Furthermore, a different liposomal formulation of E75 peptide (Lip-Pep) was injected three times into TUBO tumor-bearing mice, in combination with three injections of a liposomal formulation of Dox (Lip-Dox). The treatment with Lip-Dox and Lip-Pep promoted tumor infiltration with TILs and NK cells, stimulated IFN-γ secretion and reduced MDSCs and CD25+Foxp3+ Tregs populations in the TME more efficiently than E75 and Dox alone. Notably, mice treated with Lip-Pep+Lip-Dox showed a more significant reduction in tumor growth rates and the highest survival time [56][92].
Besides, Gp2 peptide (derived from the transmembrane domain of HER2/neu protein) was conjugated to micelles and then inserted into liposomes composed of DOPE, which contained MPL. This formulation (Lip-DOPE-MPL-GP2), used to immunize TUBO tumor-bearing mice, stimulated IFN-γ production by CD8+ T cells as well as antigen-specific CTL responses and it led to a reduction in tumor size and an increasing of the survival time of treated mice [57][93].
In addition, polymeric NPs carrying different peptides were used as nanovaccines for breast cancer. Firstly, polymeric NP, which combines MHC-I and MHC-II HER2 peptides with CpG and MPL (EntrapNP), was tested in an orthotopic HER2+ breast cancer model as a nanovaccine. EntrapNP was efficiently internalized by DCs and the exogenous antigens were cross-presented through MHC pathways. After three doses of the nanovaccine, tumor infiltration with TILs (mainly cytotoxic memory-T cells) was increased and an antigen-specific immune response against cancer cells was triggered. Treated mice showed a significant delay in tumor growth and lower incidence of metastatic lesions [58][94]. Secondly, PLGA NPs containing the designed CpG-coated tumor antigen (Tag) were shown to be avidly endocytosed and presented by APCs, especially by DCs. The encapsulated tumor antigen was composed of a membrane lysate of 4T1 tumor cells. As a result, CpG-NP-Tag NPs increased the DC maturation and activation status, stimulated the tumor-specific CTL response and attenuated breast tumor growth and angiogenesis in vivo [59][95]. In another study, PLGA-NPs were used to encapsulate Hp91, an immunostimulatory peptide derived from HMGB1. Studies in vitro demonstrated the ability of this nanovaccine to robustly activate DCs, as compared to the free peptide. Moreover, vaccination of a murine breast cancer model with PLGA-Hp91-NPs together with HER2 free peptide enhanced the activation of HER2-specific CTLs’ response, inhibited tumor development and prolonged survival time more significantly than HER2 peptide alone due to the immunoadjuvant potency of NPs [60][96].
Finally, the co-localized delivery of a nanomedicine and an antigen epitope-based nanovaccine was proposed to directly program DCs in vivo. An injectable and thermosensitive hydrogel carrying curcumin-loaded polymeric NPs (nanomedicine) and a nanovaccine was developed to completely cover the surgical bed of primary tumors and treat residual cancer cells after surgery through the sustained delivery of nanotherapy. On the one hand, curcumin NPs are able to both stimulate the recruitment and the maturation of DCs and enhance tumor immunogenicity by inducing the ICD of the residual tumor cells. On the other hand, in order to amplify the antitumor T-cell response, E75 peptide and CpG-ODN were co-assembled in a cationic polymeric NP to act as a nanovaccine. Thus, after insertion of the hydrogel in the postoperative 4T1 breast carcinoma model, the infiltration of CD8+ T cells in the relapsed tumor increased, the systemic antitumor immune response was synergistically triggered and tumor recurrence and pulmonary metastasis were significantly attenuated in treated mice [61][97].
4.3. Gene-Based Nanovaccines
Nanomedicine has been also used to improve gene-based vaccines. Several DNA and RNA-based nanovaccines are currently being evaluated as therapies for breast cancer. For instance, the delivery of a xenogeneic telomerase reverse transcriptase (TERT) DNA vaccine after the intramuscular administration of chemokine ligand 21 (CCL21) was studied. On the one hand, human TERT (hTERT) regulates cell proliferation and immortality and it is a potential TAA for immunotherapy since it is overexpressed by cancer cells. Thus, the COOH terminal part of the hTERT gene (cTERT) was fused with the PADRE sequence and a ubiquitin sequence to enhance protein presentation. The resulting DNA vector was encapsulated into liposomes with hemagglutinating virus of Japan coating. On the other hand, CCL21 is secreted in lymph nodes and contributes to the recruitment of naïve T cells and CCR7-expressing mature DCs. Hence, CCL21 injection activated DCs at the vaccination site and elicited a stronger antitumor immune response against TERT-expressing cancer cells. The combined therapy, in prophylactic and therapeutic models of TERT-expressing breast cancer, induced stronger antitumor responses based on anti-TERT specific CTLs, triggered the Th1 immune response more predominantly, and inhibited tumor growth more strongly as compared to the cTERT DNA vaccine alone [62][106].
Regarding the route of administration, oral delivery of DNA-based vaccines is a noninvasive method that has some advantages over intravenous vaccination. Alginic acid-coated chitosan NPs (A.C.NPs) were evaluated as an oral carrier for the legumain DNA vaccine in a murine orthotopic 4T1 breast cancer model due to their excellent stability, biocompatibility and capacity to enhance mucosa absorption of the drugs. A.C.NPs showed higher efficacy than the oral DNA vaccines, as they were able to resist DNA degradation in the acidic gastric environment and they increased DNA uptake and expression by APCs in the intestinal Peyer’s patches. Furthermore, as legumain is an asparaginyl endopeptidase that is overexpressed on TAMs, this oral vaccination system increased the amount of activated CTLs targeting TAMs and remodeled the immunosuppressive TME. Therefore, A.C.NP treatment inhibited activated Tregs cells in the TME, suppressed tumor growth and prolonged the survival of treated mice [63][107].
RNA-based vaccines are also being evaluated to treat breast cancer due to the two major advantages of this modality over DNA vaccines. Firstly, RNA-based vaccines can be delivered into the cytoplasm of targeted cells to produce the proteins of interest, whereas DNA-based vaccines have to integrated into the cellular nucleus to be transcribed into mRNA that will be later translated into the proteins of interest in the cytoplasm of targeted cells. Thus, RNA-based vaccines are able to produce higher levels of protein in a shorter period of time. Importantly, RNA-based vaccines are safer to use than DNA-based vaccines, since they do not integrate a foreign DNA in the genome of targeted cells. For that reason, lipid/calcium/phosphate NPs modified with mannose were evaluated to deliver an mRNA vaccine into the cytoplasm of DCs. The mRNA encoded MUC1 tumor antigen, a mucin that is overexpressed in breast carcinoma cells. The application of these NPs in combination with the anti-CTLA-A antibody into an orthotopic TNBC model induced a stronger antigen-specific CTL response against cancer cells and higher IFN-γ production, as well as a more significant tumor inhibition than monotherapies alone [64][108].
Notably, conventional DC vaccines are not therapeutically efficient in breast cancer because of the immunosuppressive TME. In order to improve the antitumor effect of DC vaccines, a strategy based on nanomedicine has been evaluated in the 4T1 murine model. The first step consisted in the intravenously administration of CD73-specific siRNA-loaded chitosan-lactate NPs to attenuate the immunosuppressive TME. CD73 is overexpressed in cancer cells and exerts immunosuppressive effects through the production of high levels of adenosine. Thus, CD73-specific siRNA-loaded NPs were able to decrease CD73 expression in tumor cells and inhibit adenosine production in the tumor site, which downregulated Treg and MDSC populations, as well as IL-10 secretion. Then, a tumor lysate pulsed DC vaccine was intradermally injected close to the tumor area. In this combined therapy, the efficacy of the DC-based vaccine was potentiated, leading to the activation of the CTL antitumor function, the stimulation of T-cell proliferation and the enhanced production of IFN-γ and Il-17. This treatment protocol inhibited tumor growth, prevented lung metastasis by attenuating the expression and activity of MMPs 2 and 9, and significantly increased survival time in treated mice [65][109].
