Intrinsically conducting polymers ICPs are oligo- or polymeric organic materials with numerous strikingly unusual properties like high electronic conductivity depending on their state of oxidation and pronounced electrochemical redox activity. Because a redox process is associated with electronic charge transfer ICPs have been proposed as charge storage materials in electrodes of secondary batteries or supercapacitors. In addition their use as binder in electrodes or as coating material has been suggested. ICPs was briefly introduced and these various applications in batteries were highlighted here.
- intrinsically conducting polymers
- oligomers
- conjugated molecules
- secondary batteries
- electrochemical energy conversion
- electrochemical energy storage
1. Introduction
In electrochemical devices for energy storage electric energy is stored by electrochemical transformation (electrode reaction) of electrode materials (active masses) from a state of lower energy (corresponding to a lower value of Gibbs energy or free reaction enthalpy with respect to the device containing two electrodes; frequently stated data with respect to only one material or electrode are meaningless) into a higher state (commonly called charged state). The option of storing electric energy without such conversion in the electric field of a capacitor or the magnetic field of a coil has become relevant for the first option only recently with the advent of supercapacitors, the second option is relevant only for storage on a small scale for a few special applications. Figure 1 illustrates these options schematically.
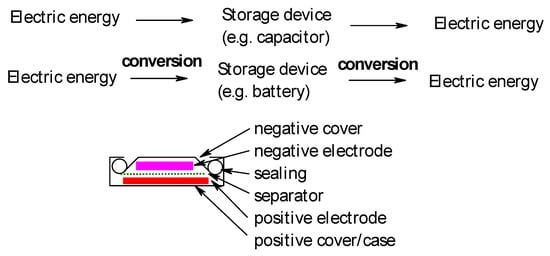
Figure 1. Basic modes of electric energy storage (top), cross section of a secondary battery, coin-type (bottom).
The associated electrode reactions should proceed fast and reversibly (in its various common meanings: close to equilibrium conditions without excessive losses due to electrode overpotentials caused by slow electrode reactions, in both directions on the same reaction pathway), they should be possible in both directions as many times as desired by the user without significant deterioration of the participating materials [1][2]. Numerous materials of mostly inorganic origin have been examined, many of them are commonly employed in commercial products [3]. Organic materials have been considered only infrequently, presumably because of concerns about insufficient stability and about inferior charge storage capability. An overview of typical data (Table 1) comparing storage capabilities of inorganic and organic materials corrects the latter concerns:
Table 1. Selected electrochemical data of some electrode materials.
| Material | Molecular Weight of Repeat Unit/g | Oxidation Level */- | Theor. Q # | Measur. Q/F·g−1 |
|---|---|---|---|---|
| PANI | 93 | 0.5 | 750 F·g−1 | 240 |
| PPy | 67 | 0.33 | 620 F·g−1 | 530 |
| PTh | 84 | 0.33 | 485 F·g−1 | - |
| PEDOT | 142 | 0.33 | 210 F·g−1 | 92 |
| Porphyrin C20H14N4 |
310.35 | 1 | 311 As·g−1 | - |
| Quinone/HQ | 108 | 2 | 1787 As·g−1 | - |
| Ferrocene | 185 | 1 | 522 As·g−1 | - |
| Li | 6.939 | 1 | 13,904 As·g−1 | - |
| Al | 26.98 | 3 | 10,728 As·g−1 | - |
| PbO2 | 239 | 2 | 807 As·g−1 | - |
1 Data taken from [4]. PANI = polyaniline, PPy = polypyrrole (also poly (2,5-pyrrolylene)), PTh = Polythiophene (also poly (2,5-thienylene)), PEDOT = poly-3,4-ethylenedioxythiophene. * Oxidation level, also “dopant level”, reports the fraction of oxidized repeat units and the number of electrons transferred in the electrode reaction. # gravimetric charge density can be stated with respect to the electrode reaction in units As·g−1 or in case of a material where no clear electrode reactions can be stated as an amount of charge stored within a change of electrode potential in units of As·g−1 (i.e., F·V−1·g−1).
Numbers reported in the representative selection of Table 1 should be considered with care when referring to ICPs. In case of materials with well-defined discrete charge-storage sites like lead ions in PbO2 or aluminum atoms in an aluminum electrode in case of ICPs charge transfer may proceed at a given location of the polymer chain, but the created charge is delocalized along the molecular chain leaving the question along how many repeat units delocalization is effective. The answer is stated with the oxidation level. At a level equal to 1 all repeat units are oxidized (reduced), but real values tend be much smaller as discussed below. Such a state may be reached with PANI when the pernigraniline state is established (for more details see below), but this state is chemically rather unstable, it is commonly called overoxidized with respect to the application of ICPs presented here. Overoxidation of PANI as well as other ICPs has been studied, but as deplored elsewhere [5] no review has been provided so far. It appears safe to state at this point that increase of storage capability by applying lower/higher electrode potentials will most likely result in faster electrode and consequently battery degradation.
A further comparison of electrode materials as shown in a representative selection only in Table 1 must address its ionic and electronic conductivity. Both modes of charge transport depend on concentration and mobility of charge carriers. At first glance metallic electrodes (e.g. lead in the lead acid battery) appear to be almost ideal. Real electrodes even of lead or zinc are porous in order to provide a large surface area. The effective electrical conductance will be decreased. The majority of battery electrode materials – both organic and inorganic ones – are poor conductors, semiconductors or almost insulating. Whether the poor electronic conductance of a material is due to low concentration or mobility depends on many factors, in case of inorganic materials crystallinity, impurities, morphology are among the relevant factors. In case of organic materials details of molecular structure, degree of polymerization and molecular ordering can be relevant, an overview provides more insights [6]. Ionic mobility is relevant when chemical reactions and ion transport phenomena are connected to the electrochemical redox reaction as the central step in energy conversion and storage. Mobility of lithium atoms and ions in graphite is one of the factors limiting current generation capability of this negative electrode in lithium-ion batteries, the same applies to intercalation or insertion compounds serving both as negative or positive electrode in metal-ion batteries. With organic materials such connected reactions are less frequent, in reports on the performance of organic electrode materials including those presented below limitations related to ionic mobility are rarely – if at all – addressed. In a wider sense mobility of ions acting as counter ions for charge balancing may also be relevant. Ions moving in or out of the porous electrode material slower or faster may negatively affect the possible current. Studies addressing explicitly this issue are lacking. Even without exact knowledge of conductivity-related material parameters electrode design (for a scheme see below) attempts to take care of the related issues more or less intuitively.
Further advantages and also limitations of organic materials, in particular ICPs, as battery electrode materials will be addressed in detail below.
2. The mMaterials: Intrinsically cConducting pPolymers
The term intrinsically conducting polymer designates a class of mostly organic oligomeric or polymeric materials which show electronic conductivity because of mobile charge carriers capable of moving along conjugated segments and hopping between such segments. Materials afforded with electronic conductivity by addition of an electronically conducting material like graphite powder or metal fibers to an insulating material are simply and unfortunately somewhat misleadingly called conducting polymers, their other designation as filled polymers is not better and as imprecise because it addresses the addition of a second material (forming a composite) which may be insulating and/or may provide some other functionality.
Because of this amazing merger of typical properties of an organic material with those of a metal, for example, a very inorganic material, ICPs have sometimes been called synthetic metals. Unfortunately the latter term has also been applied to a class of crystalline materials: charge transfer complexes (for example, N,N'-dicyanonaphthaquinonediimine (DCNNI) and tetrathiafulvalene (TTF). This has finally resulted in some confusion and frustrated readers guided to reports on such materials [7][8] when expecting materials for energy storage in ICPs.
ICPs are composed mostly of carbon and hydrogen; sulfur, nitrogen and oxygen may be present as heteroatoms. A selection of typical examples with simplified molecular structures, systematic names and generally accepted acronyms and typical values of electronic conductivities is provided in Figure 2. More examples can be found in monographs and handbooks [9][10][11][12][13]. Because of their fascinating combination: organic matter with metal-like properties ICPs have attracted tremendous research activities once in particular the electronic conductivity was noticed; but actually they have been known much longer (for brief historic overviews see [14][15]. Among the numerous applications of these oligo- and polymeric materials showing conjugation already in their repeat units and even more extended conjugation along the molecular chains their use as active materials in devices for electrochemical energy storage and conversion was suggested early. Initially this meant use in primary and secondary batteries, with the advent of supercapacitors their use in these systems was examined also. It was focused on secondary batteries, but given the ongoing merger of batteries and supercapacitors noticed before [16] and reviewed elsewhere [17] considerations and arguments apply frequently also to supercapacitors; materials may turn up in both types of devices.
For overviews on selected aspects of the application of ICPs in supercapacitors see [18][19][20][21][22][23][24][25][26] their use in flexible devices has been discussed in [27][28], in stretchable devices in [29].
A different class of polymers with a pronounced electrochemical redox activity is called redox-polymers [13]. In these polymers localized redox-active functional entities, mostly substituents (or pendant groups) at a molecular backbone showing neither own electrochemical activity nor conjugation enabling electronic charge transport along the backbone, provide an electrochemical response which might indeed also be of practical relevance for electrochemical energy conversion and storage. In case these moieties are attached to the backbone of an ICP they will possibly disturb conjugation, diminish the ICPs capability to stabilize mobile charge carriers and thus turn the ICP into a poorly conducting polymer [30]. This can possibly be avoided by substitution with ionogenic moieties showing no redox functionality like sulfonate groups [31], for examples see below. Because they differ chemically very much from ICPs and because their mode of operation is very different also they are not treated here, reviews are available [32][33][34]. Sometimes these polymers show electric conductivity enabled by charge transfer between these redox centers by charge hopping. Such polymers are called redox-conducting polymers [35], they are also beyond the scope here.
ICPs can be prepared by a variety of polymerization methods with widely varying features more or less suitable for a particular application, for an overview see [36]. Preparation conditions and experimental parameters can be adjusted yielding specific morphologies [37], for more examples and details see below.
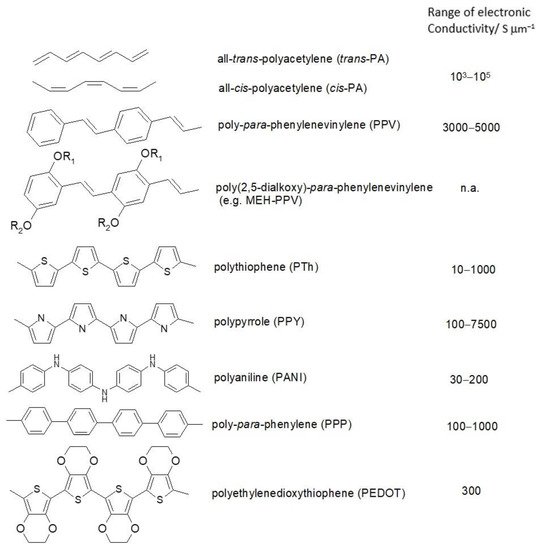
Figure 2. Examples of intrinsically conducting polymers.
3. The aApplication: Intrinsically cConducting pPolymers in sSecondary bBatteries
ICPs have been suggested as constituent in secondary batteries, in particular in electrodes, in various functions. These are briefly addressed below, selected applied ICPs will be presented thereafter.
3.1 Use as aActive mMass
Initial optimism regarding real applications of ICPs in numerous fields including the subject of this entry here has been marred by either observed or at least expected insufficient lack of long-term stability resulting in electrochemical applications in turn in decreasing storage and current capability. In general terms this has been addressed for ICPs in secondary batteries in [1] and for supercapacitors in [38]. The sometimes sloping discharge cell voltages were sometimes claimed as a further drawback. The characteristic property of every ICP, its electronic conductivity, changes as a function of the state of oxidation/reduction of the ICP across several orders of magnitude (for example, from about 107 S·m-1 for doped, oxidized trans-PA to 10-2 S·m-1 for neutral, undoped trans-PA). Sometimes (for example, PANI) protonation plays an additional role (at pH = - 0.3 the change covers four orders of magnitude, at pH = 7 only three orders, in organic electrolyte solutions even less). This fascinating property is an unwelcome one in the application discussed here because low conductance tends to limit current capability of an electrode material and to increase the Ohmic resistance of electrode material and complete cell. The rather uninspired and fairly traditional approach towards a compensation of this is the addition of conducting materials like carbon black or graphite. Unfortunately, this adds dead weight and decreases specific storage capability. Another approach addressed below is the use of thin films on highly conducting substrates or 3D-architectures.
The recent surge in interest in ICPs for such applications is presumably related to the interest in materials with practically unlimited resources (which is a growing concern with many current battery materials), to the possibility of rather simple handling of used/worn out materials not requiring the specific procedures required for handling heavy metal-containing batteries and devices, to the simple redox reactions not encumbered by intercalation or other possibly slow reaction steps, and to mostly smaller energy usage in preparing these materials under environmentally acceptable conditions (for a broader overview with regard to metal-ion batteries see [39]). Changes in materials properties can be afforded in most cases using the tools of well-established organic synthesis [30]. Finally these materials may provide a bridge to the use of renewable materials like lignin [40][41][42][43][44][45] or other materials derived from natural resources [46][47][48]. Lastly these materials may enable or at least simplify construction of flexible and even stretchable devices. The pronounced conjugation in ICPs, which even changes as a function of the degree of oxidation/reduction, stabilizes charges on the chain, it also results in strong interactions between sites where oxidation/reduction proceeds, this is one of the causes of sometimes poorly developed current peaks in CVs [49].
Lacking compatibility with established systems in terms of cell voltage as another barrier initially observed does not appear to be a problem anymore with batteries installed in electronic devices without an option of simply exchanging them like with standard batteries before. Non-standard cell voltages and other specific features of a given cell thus can be easily integrated into the device. The current level of interchangeability of devices and batteries (with cell voltages of mostly aqueous systems around 1.5 V) is thus lost, but this appears to be no major concern anymore in many cases. Instead, perspectives of all-polymer or even paper-based batteries appear to be more attractive or even realistic [50].
Charge storage in every material proceeds by redox reactions. Another possibility – charge accumulation in the electrochemical double as employed in electrochemical double layer capacitors EDLC [51][52] – is also of considerable interest but not in the focus of this contribution, it is not related to conjugation in molecules and the associated electronic conductivity. Because an electrode with an ICP as active mass will establish a double layer with the associated double layer capacity when brought into contact with an electrolyte solution capacitive contributions to charge storage and with regard to the full cell to energy storage may be important nevertheless. How to separate these contributions is the subject of an ongoing heated debate [53], specific aspects with regard to PANI have been reviewed recently [54].
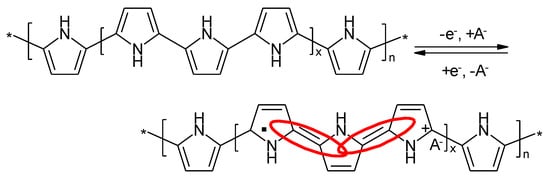
In case of an ICP charge transfer proceeds via removal (oxidation, most frequently) or addition of an electron (reduction, less frequently observed because of the relatively low electrode potentials needed in most cases and the poor stability of the created species) yielding a radical cation in the former and a radical anion in the latter case. The radical property is caused by the fact, that removal from an electron in a HOMO leaves an unpaired electron; addition of an electron into a LUMO also yields an unpaired electron. This radical property has been extensively probed with electron paramagnetic resonance spectroscopy [55]. The term doping, p-doping in case of the oxidation and n-doping in case of a reduction, is frequently used. Different from the quite common meaning in semiconductor physics where doping means substitution of atoms (e.g. silicon or germanium) by very small quantities of (dopant) atoms with different valencies (e.g. boron, gallium, phosphorus causing hole or p-conduction in case of the group 3 elements and n-conduction in case of the group 5 element) with ICPs apparently refers to the type conduction at first glance: hole conduction upon oxidation, electron conduction in case of reduction. Whether hole conduction actually means electron movement into the opposite direction is perhaps a more philosophical question in the present context. In any case the numbers (correctly number densities or concentrations) of generated charge carriers is larger by orders of magnitude as compared to semiconductor doping of e.g. silicon. Adding to the confusion is the further use of dopant ion to designate counter ions moving in or out of the ICP for charge compensation. In this entry neither generation of further confusion nor imprecision are intended, terms related to doping are avoided.
Because of conjugation in the studied oligo- or polymer this electron (or hole) is not located, the observed EPR-spectra show single lines and no hyperfine structure. This delocalization provides also the electronic conductivity along the molecular chain as a typical feature of an ICP. Movement of charges between molecular chains is provided by electron hopping. Removal of more than one electron is possible, accordingly there may be no unpaired electrons anymore, the radical property is lost as evidenced again with EPR-spectroscopy [55]. As a consequence of the delocalization of the generated charge(s) different from redox processes with metal ions no well-defined redox potential is created, the electrode potential and consequently the cell voltage change more or less continuously as a function of transferred charged, this is generally called sloping discharge voltage.
These processes can be envisaged with PANI as shown in Figure 3.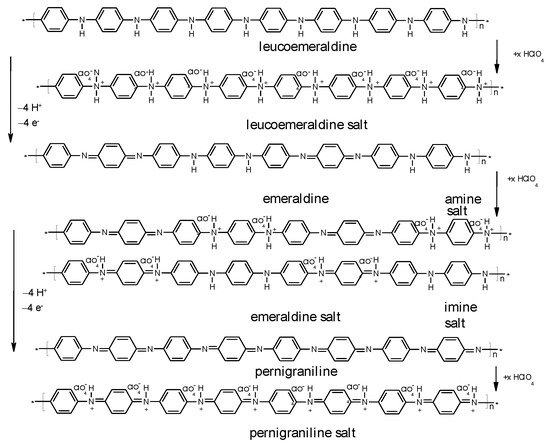
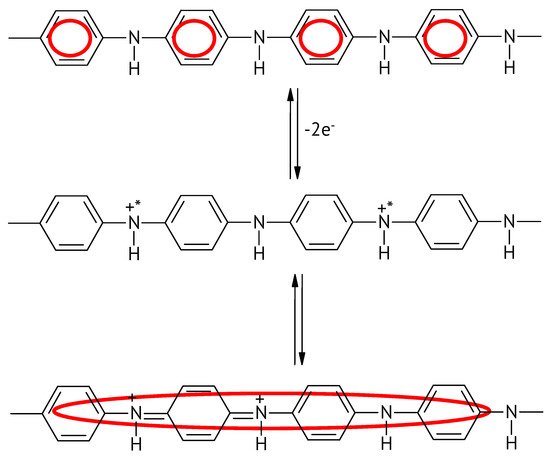
Figure 4. Changing extent of conjugation upon the oxidation of the emeraldine form of PANI.
The actual extent of conjugation may differ. Determination of molecular weights of ICPs is notoriously difficult; in addition actual values may differ wildly for a given ICP depending on preparation conditions. This extent should not be confused with the effective conjugation length introduced by Zerbi et al. [56].
The associated electrochemical response of a film of PANI is shown in Figure 5.
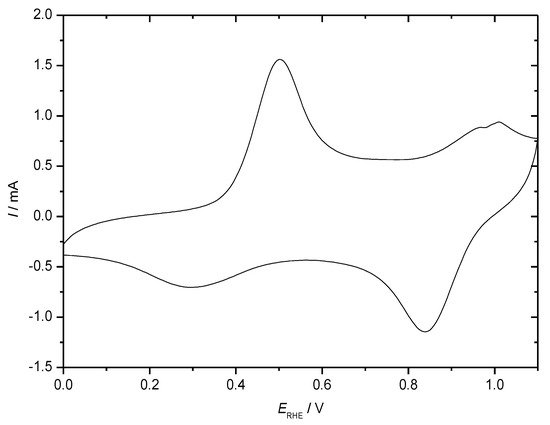
Figure 5. CV of a polyaniline-coated (electropolymerization by 300 electrode potential cycles within the potential range as indicated) stainless steel grid electrode (1 cm2) in an aqueous electrolyte solution of 0.1 M aniline + 1 M HClO4, dE/dt = 100 mV·s−1, nitrogen purged.
In the positive-going electrode potential scan in a cyclic voltammogram the first redox transition from leucoemeraldine to emeraldine is observed as the current peak around ERHE = 0. 5 V, the second peak indicating the further transition to pernigraniline around ERHE = 1 V is not very pronounced because electrode excursion into this region (overoxidation) causes diminished chemical stability of the polymer resulting in subsequent degradation. In the subsequent negative-going scan both processes are reversed. The distinctly different peak shapes are due to the different electrochemical and structural situations: When starting at ERHE = 0. 0 V PANI is in its poorly conducting (semi-conducting possibly depending on the definition of this term [6]) state providing hardly electronic communication between redox sites where electron transfer proceeds resulting in a well-developed peak [49]. The second peak recorded with PANI already in its highly conducting emeraldine state is sometimes much broader (as observed here) because of the extensive electronic interactions between redox sites supported by the extended conjugation along the molecular chain. Depending on preparation conditions [57] , film thickness, morphology and so on. different results can be observed as shown e.g. in [58] with a thin film on a gold electrode providing a well-developed second peak. In the negative going return scan the oxidation processes are reversed, the same arguments as made before can be applied to peak shape. In addition to the more or less pronounced current peaks a rather large residual current between the peaks can be observed. Assignment to a double layer charging current or to a redox process has been the subject of extensive research and discussion; some considerations have been collected in [49]. Because ICPs show frequently an irregular morphology suggesting a large surface area of the material in contact with the electrolyte solution considerable values of a double layer capacitance can be expected, this is of particular interest for high-current applications (like in supercapacitors), where the fast charge/discharge of the double layer sustains much higher currents than the relatively slow redox processes. Because of the relatively fast discharge of EDLC supercapacitors due to dissipation of the accumulated charges in the double layer [59] this contribution is less relevant for secondary batteries considered here. The frequently claimed high fraction of capacitive/pseudocapacitive contributions in battery electrodes is most likely based on a wrong model as briefly discussed before [53] and pointed out initially elsewhere [60]. Electrochemical impedance measurements may provide an approximation to the desired separation [61][62].
Closely associated with these redox processes are further changes possibly resulting in deterioration of the ICP and in case of an electrode with an ICP as active mass in electrode performance.
Shape change
The redox processes as shown in Fig. 2 are associated with ion movements. For charge balancing upon oxidation either negative (an)ions have to move into the film or cations (e.g. protons) have to move out. The latter is possible only with polymers having molecular sites which can be protonated/deprotonated. Whether ion ingress and/or egress proceed and which process possibly dominates has been studied extensively, for an introduction see [63]. These processes are associated with volume changes (swelling/shrinking, also: shape change) of the polymer because the ions move with some solvation shell. This may negatively affect stability and performance. These changes may result in fragmentation of the polymer, loss of contact between ICP particles, added conducting carbon and the current collector. Various options to mitigate this shape change have been proposed and examined:
Malinauskas has suggested self-doped ICPs, e.g. self-doped PANI [64]. In such polymers the presence of fixed negative charges located on anionic groups changes the mechanism of charge compensation during the redox processes of PANI: instead of ingress of anions during oxidation possibly associated with the problems discussed above release of cations (e.g. protons or lithium ions) bound at the anionic sites suffices for charge compensation with less detrimental effects. Such self-doped PANI can be obtained in various ways:
- Sulfonation of PANI by chemical post-treatment.
- Polymerization of suitable substituted monomers.
- Copolymerization of aniline and a second suitably substituted comonomer (e.g. copolymerization of aniline and e.g. N-methylaniline and N(3-sulphopropyl)aniline [65]] (see Figure 6) or aniline and o-aminobenzene sulfonic acid [66] or m-aminobenzoic acid and aniline [67][68]).
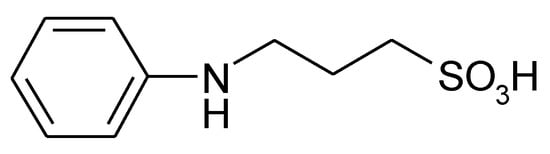
A copolymer prepared from aniline and N(3-sulphopropyl)aniline (for examples see [65][69]) has been studied. In the former study with N-methylaniline as the comonomer highest electrochemical activity was observed when a comonomer ratio of 1:1 was established in the electropolymerization solution. This agrees quite well with the degree of doping 0.5 listed above for PANI in Table 1 with half of the repeat units formally participating in the redox reaction. Somewhat surprisingly this approach has not been exploited in reported research elsewhere. In an investigation employing a copolymer of aniline and m-aminobenzoic acid as positive electrode 30 % capacitance loss after 1000 cycles for a complete supercapacitor cell with a PPy negative electrode were noticed, unfortunately no comparison with a similar system without a self-doped ICP was attempted [70]. The copolymer obtained from aniline and metanilic acid by electropolymerization showed significant electrochemical activity in a neutral aqueous electrolyte solution of 0.5 M Na2SO4 with about 50 % capacitance retention when assembled into a symmetric supercapacitor [71].
Because of the pH-sensitivity of a zinc electrode, which is hardly compatible with an acidic aqueous electrolyte solution preferred for plain PANI, self-doped PANI has been suggested as a form of PANI without the need for an acidic electrolyte solution for a rechargeable zinc-ion PANI battery [72]. As a compromise a mildly acidic electrolyte solution (pH = 4.5) has been suggested for a PANI/zinc cell [73]. Deposition of PANI as a thin film on an electronically highly conductive 3D-substrate further assisted in ameliorating the problem of moderate electrochemical activity at this pH [74].
The concept of self-doping has rarely been explored beyond the particularly pH-sensitive PANI. An attempt to prepare an oligomeric bis[3,4-ethylenedioxythiophene]3thiophene butyric acid has been reported [75], its use in “green energy” application has been proposed but not explored.
Another approach examined more frequently is the formation of micro- and nanostructures allowing shape and volume change to an extent large enough to keep the operation capability of the ICP at still acceptable performance of the material. A comparison of various morphologies (2D: thin film; 3D: microsphere, microtube, microparticles, nanowires networks, nanowire arrays) in particular for supercapacitor electrode applications has been provided [24], nanowire arrays have been suggested as the most promising approach. A similar overview focused on secondary batteries is available [76]. A broader overview focused on 1-D structures of ICPs was provided [77]. In addition to providing more stable electrode structures (morphologies, architectures) nanostructuring may also help to improve performance in terms of current capability. A growing current across the electrode/electrolyte solution interface at a constant interfacial area results in larger deviation of the electrode potential from its equilibrium value, a larger overpotential. This unwelcome effect can be limited by increasing the interfacial surface area using a porous electrode or another 3D-structure. Bottom-up strategies starting with molecular entities assembled in a controlled way into suitable 3D-structures are available as well as top-down strategies starting with bulk materials transformed by milling, evaporation-deposition and so on into suitable 3D-structures. For ICPs obviously the former approach is appropriate, details and examples are presented below. A further concern comes into play: For increased energy of a cell and larger storage capability of an electrode more material, for example, a thicker electrode, is required. As illustrated schematically in Figure 7 more mass and current capability limited by Ohmic resistance of the electrode material and the electrolyte solution must be taken into account.
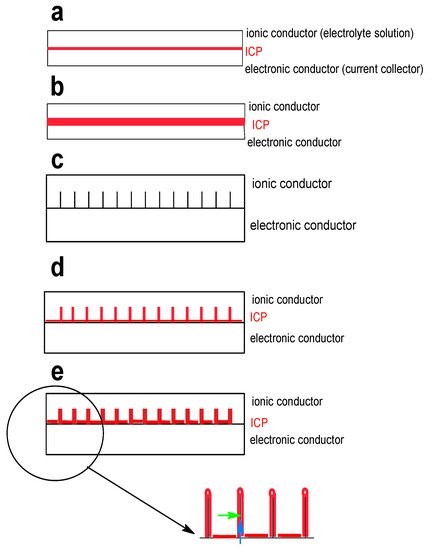
A thin electrode (Figure 7a) may not provide enough mass, a thicker non-porous electrode (Figure 7b) has longer electronic pathways when the electrode reaction still takes place at the ICP/solution interface, an electronically conducting 3D-support (Figure 7c) provides larger interfacial area to be covered subsequently with active mass (Figure 7d), the actual coating must finally balance electronic and ionic conduction pathways and their respective lengths and contribution to Ohmic resistance (Figure 7e and insert). 3D-supports (instead of smooth metal foils) can be metal grids or meshes, carbon or graphite paper or carbon structures prepared by pyrolysis of natural materials from biological sources. Further structuring on an even finer level can be afforded by controlled and directed ICP deposition. These considerations also apply to materials and electrodes for supercapacitors and they have been highlighted before [78]. Possible options for various ICPs will be addressed below.
Two further modes of ICP and thus electrode deterioration have been noticed and examined in detail:
Peeling off
Closely related and particularly relevant for electrodes where the ICP has been directly deposited onto the current collector (for example, no binder is used) is peeling off from the current collector leaving the removed particles lost for charge/discharge. Because it is closely related to shape-change, actually may be a result of it, remedies against the former may help against peeling off.
Overoxidation
Overoxidation is closely associated with charge/discharge of an ICP in a battery electrode. When the electrode potential of an ICP is moved into positive direction (see Figure 5) electrooxidation forms cations on the polymer chain, chemically speaking they are radicals (polarons is another designation borrowed from solid state physics). These species can be subject to chemical transformation by reaction with electrolyte solution species. When sufficiently positive electrode potentials are reached additional oxidation of water may yield radicalic intermediates which in turn chemically attack and degrade the ICP. The anions moving into the ICP in most cases for charge compensation may affect these reactions, studies of anion-specific effects show such influences for PANI [79]. Reaction mechanisms and effects of overoxidation vary, and in most studies decreases in possible electronic conductance, changes of molecular structure, decrease of possible charge storage and in the extreme case complete loss of electrochemical redox response are reported (for examples see [80][81]). Unfortunately, a wider review on this subject seems to be missing, only the degradation of some forms of PEDOT has been inspected more closely in an overview [82]. At first glance a simplistic view would suggest an easy solution by limiting the potential excursion with suitable electronic circuitry providing limitation of maximum charge voltage. Unfortunately, practical execution is more complicated. Although the operating voltage limits of batteries have always required appropriate circuit design considerations the rather low operating voltage of a secondary battery or a supercapacitor with an aqueous electrolyte solution and the sensitivity towards overoxidation require more precise voltage limitation making the use of batteries and supercapacitors with these materials less attractive. In addition, potential distribution inside of an electrode may leave parts of the material exposed to electrode potentials already too high causing overoxidation. Consequently, other options have been examined (for example see [24]). Selection of suitable solvents and electrolytes may help to avoid oxidative formation of chemically reactive species (like hydroxyl radicals when water is used as a solvent).
The redox processes discussed above with PANI as an example are one option of charge storage in addition to double layer storage. The associated ion movement is basically not related to any redox process at the other electrode, there is even no need that any participating ion is involved in reactions at both, i.e. the negative and the positive electrodes (Frequently the former electrode is called the anode, the latter one the cathode. Obviously these terms are correct only during discharge; during charge the anode turns into a cathode with considerable possibilities of confusion [83]. Consequently, this terminology should be avoided). In case of the very popular metal-ion batteries (for example, lithium-ion or sodium-ion battery) ions, mostly the metal cations, move between the electrodes. This has caused the nick-name “rocking-chair battery”. Their major advantage is the almost completely constant concentration of ions, i.e. cations, in the electrolyte solution keeping the ionic conductivity and thus the Ohmic internal resistance of the battery constant. Accordingly the ICP operating in an electrode in such a cell should provide sites for metal ion storage. At this point the more or less conjugated system of an ICP is not helpful for providing storage sites by itself. Instead ICPs with attached functional groups have been proposed. An overview is provided in [5], and representative examples will be given below.
It is about various aspects of ICPs as applied in electrochemical energy conversion and storage are available [19][30][78][83][84][85]. This includes the particularly frequently studied use of ICPs as host material for sulfur in metal-ion sulfur batteries confining soluble polysulfide species and suppressing the shuttle mechanism, for overviews on this subject see [5][86].
3.2 Use as bBinder
With very few exceptions (lead or lead dioxide electrode) addition of a binder to the active mass for an electrode is required to provide cohesion between electrode particles and to provide adhesion to the electrode support and current collector. Conventionally the use of synthetic polymers added with several percents of weight is the standard. These binders are electrochemical inactive and electrically insulating, they decrease the specific storage capability of the electrode masse and negatively affect the conductivity of the electrode and in turn the current capability. ICPs have been proposed as substitutes which form in additional coatings on active particles (core-shell structures) supporting improved performance. In particular PEDOT has been studied as a binder for electrodes in lithium-ion batteries. An overview describes advantages and already achieved performance [87]. The adhesive properties of this ICP provide enough cohesion to maintain integrity of the active material, in addition their electronic conductivity may enhance current capability. The additional transport pathways are schematically illustrated in Figure 8.
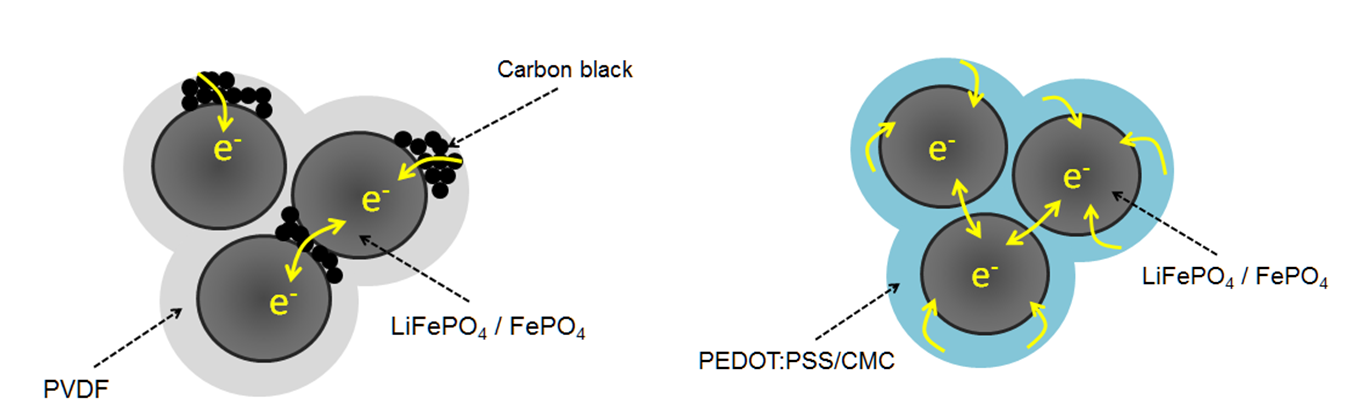
Figure 8: Electron transport in a positive electrode of a lithium ion battery with added carbon black and a polymeric binder (left) and with PEDOT:PSS as a binder (right) [87].
3.3 Use as cCoating
Some electrode materials show attractive storage capacities but insufficient stability. This may be due to volume changes during redox processes resulting in mechanical disintegration, this may also be due to solubility of the material at least in one state of charge. Coating with ICPs has been tested successfully. A polypyrrole-coated LiV3O8-nanocomposite for use in a lithium-ion battery with an aqueous electrolyte solution schematically illustrated in Figure 9 has significantly improved long-term cycling performance [88].
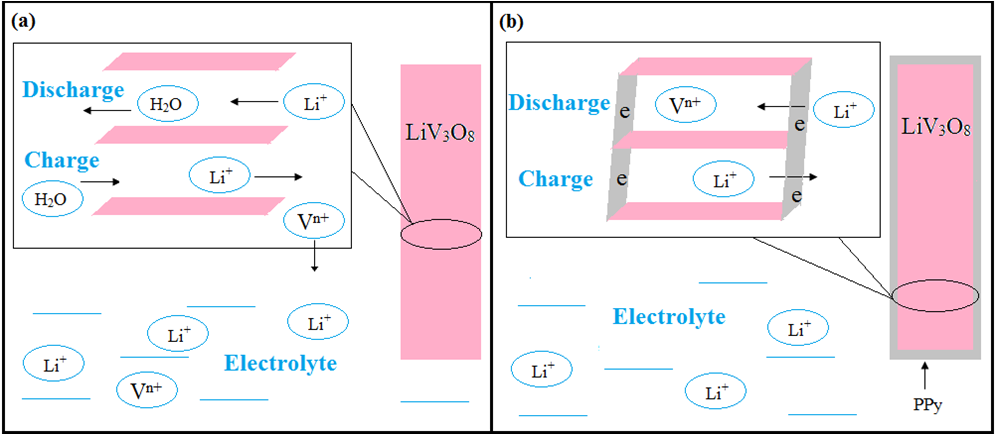
Figure 9: Proposed mode of action of a PPy-coating on a LiV3O8-nanocomposite [89].
3.4 Miscellaneous uUses
Coating of metal foils as employed in many metal-ion batteries as mechanical support and current collectors with various ICPs to improve material adherence and to afford corrosion protection has been proposed [87].
4. Selected pPolymers
Here are some representative ICPs proposed and/or actually studied for application in secondary batteries are briefly presented and typical application examples and obtained results are included. For overviews see also [90][91][92].
4.1 Polyaniline
Possibly the first secondary battery with a PANI-based positive electrode has been reported in 1990 [93][94]. In the coin-type cell with a nominal cell voltage of 3 V the negative electrode was prepared by electroplating lithium on aluminium, the positive electrode was PANI electropolymerized on a metal mesh, a mixture of propylene carbonate and 1,2-dimethoxyethane with LIBF4 served as electrolyte solution. Reasons for their rather disappointing marketing have not been reported, it might be speculated that – like with PPy (see below) – lacking interchangeability and risks inherent with a metallic lithium electrode in a secondary battery may have been factors.
To improve performance of PANI in a metal-ion battery (e.g. sodium-ion) sulfonated PANI (see Figure 10) has been proposed as sodium storage material [31][95], as discussed above this might also be a route to stabilization of the ICP by reducing the effects of shape change associated with counter-ion movement.
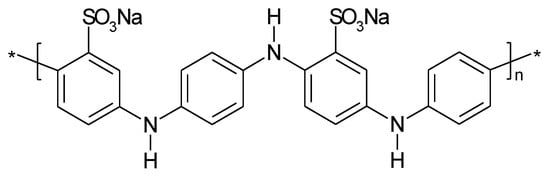
Instead of self-doping by suitable substituents the use of e.g. PEDOT:PSS (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate) can introduce the same effect: avoid deprotonation of PANI and loss of electrochemical activity [96]. A nitro-group introduced by chemical copolymerization of aniline and nitroaniline has been proposed as a further option [97].
Nanostructuring of PANI already during formation by mostly chemical polymerization into e.g. nanofibers, nanotubes, nanorods and their arrays on suitable supports (see above) has been addressed, for overviews and examples see [84][85][97][98][99]. Effects of template materials (both hard and soft) on obtained nanostructures are illustrated in the following microscope images (Figure 11).

PANI and its composites as active mass in electrodes for further metal-ion batteries have been evaluated [101]. Not suffering from the mismatch of suitable pH-values for PANI and a zinc electrode in an aqueous electrolyte solution addressed above combination of PANI as negative electrode with a positive PbO2-electrode yields a type of rocking-chair battery with anions (sulfate) moving between the electrodes [102].
PANI in supercapacitors has been reviewed earlier [103][104]. Further aspects of PANI in electrochemical energy conversion and storage have been discussed before [105].
Vanadium oxygen hydrate intercalated and exfoliated with PANI has been examined as positive electrode material for an aqueous zinc-ion battery has been studied [106]. Electronic conjugation of the PANI backbone and the conceivable electrostatic interaction of the conjugated π-system with Zn2+-ions and O2—-ions of the V-O-layers have been suggested as cause for the growth of the measured effective diffusion coefficient of Zn2+-ions by a factor of 10 to 100.
4.2 Polypyrrole
The redox processes in a PPy-electrode schematically shown in Figure 12 lack the noticeable pH-dependency observed with PANI in aqueous electrolyte solution.
A first cell with two PPy electrode has been described [107]. Although high Columbic efficiencies were noticed poor charge retention – an essential for secondary batteries – was a major drawback, the high Ohmic resistance of PPy in the neutral state caused a large internal resistance of the cell: a further drawback. Presumably the first secondary metal-ion battery with a PPy-film 50 μm thick as positive electrode, a lithium metal foil 100 μm thick and an electrolyte solution of 0.5 M LiClO4 in propylene carbonate has been reported in 1987 [108][109][110]. The open-circuit cell voltage when fully charged was 3.6 V, operational cell voltages between 2.0 and 4 V were recommended. At the latter value presumably overoxidation of the positive electrode might have become significant; in addition safety problems related to dendritic lithium deposition may have been one more argument preventing a commercial success of this system. At the time of development the already mentioned problems of cell voltage incompatibility might have contributed to the commercial failure of this attempt. In a comparative study PPy as positive electrode with a metallic lithium negative electrode has been identified as relatively more stable than PANI and PTh [111]. The same conclusion has been reported elsewhere [112].
Polypyrrole nanopipes have been proposed as positive electrode materials for lithium-ion batteries [113]. They combine high rate capability because of the established structure combined with improved storage capability in comparison to e.g. activated carbon used for high rate lithium-ion systems (batteries and capacitors) and mechanical stability.
Electrochemical procedures towards some PPy nanostructures have been developed [114], further aspects of nanostructures of PPy as obtained by electrodeposition have been reviewed elsewhere [115]. PPy nanotubes have shown particularly high electronic conductances [116][117], this in turn is advantageous when they are applied as battery electrode material.
4.3 Polythiophene
First attempts to use electropolymerized PTh as electrode material for secondary batteries have been reported by several groups starting in 1982 [118][119][120]. The redox process of the oxidation process is schematically shown in Figure 11, the reduction process yielding a radical anion instead of the cation as shown in Figure 13 is analogous.
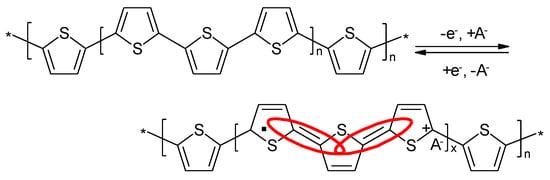
In case of PTh the ICPs obtained via electropolymerization (as the ones discussed first) and by chemical polymerization showed striking differences [121]. It was suggested a different polymer sequence in another report. (Figure 14).
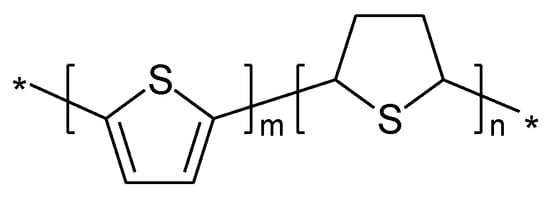
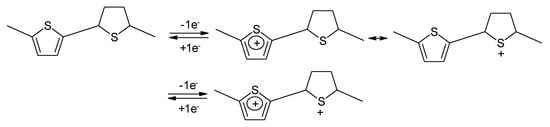
Strictly speaking this polymer is not a conjugated one. As a consequence it was suggested a sulfur-localized redox behavior also being the possible cause of the less sloping cell voltage. The reaction is schematically illustrated in Figure 15 [121]:
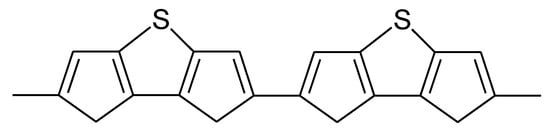
For further details on chemically prepared PTh and its use as a battery electrode see [122]. An all-PTh battery has been reported [123][124]. Slightly higher doping levels than 0.33 reported for plain PTh (see Table 1) were observed with polydithienothiophene (Figure 16) [125][126][127].
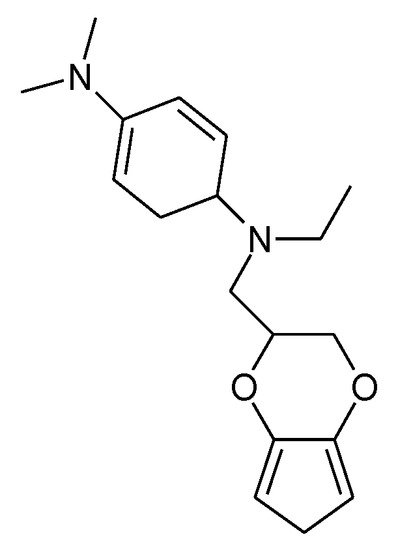
Closely related to PTh is the ICP PEDOT (see Figure 2). It has been examined with respect to its suitability as an electrode material [128] revealing exceptional stability of this ICP in its oxidized form even in aqueous electrolyte solutions [129], more frequently in electrochemical energy storage appears to be its use as a binder (for an introduction see [5]). With further substitution by N,N,N’,N’-tetraalkylated-p-phenylenediamine (see Figure 17) an ICP showing a charge transfer of up to 2.6 e- per repeat unit could be obtained with electropolymerized material [130]. This corresponds to two redox waves observed in CVs. Possibly the majority of transferred electrons go into the substituent, following Table 1 a minor fraction might go into the polymer PTh backbone.
Beyond the three ICPs considered above in detail as representative examples and parent molecules for whole families of substituted molecules (sometimes misleadingly called derivatives) further ICPs have been suggested, and some of them like polyindole [131] have been reviewed with respect to their use in secondary batteries. More ICPs and further aspects have been addressed on the use of ICPs in secondary batteries and so on [5][25][132][133][134][135]. Polymers including ICPs to be used as active masses in metal-ion batteries have been compared [136]. Systems with polyacetylene – sometimes considered as the most basic ICP and among the first to be suggested in an all-polymer battery[137] – have been examined, results appear to go barely beyond academic interest. For an overview see [83].
5. General aAspects and cConsiderations
With essentially organic polymeric materials as active masses in the electrode(s) an all-polymer battery appears to be feasible, an example of an all-PTh battery has been mentioned above. As pointed out reduction of an ICP – this would be necessary to generate the charged state of the negative electrode – is of limited practical value when possible at all. Frequently the reduced state containing radical anions is not sufficiently stable chemically; whether their claimed [30][138] “large impedance” is a further limitation remains unclear. At first glance already a device having the same material in both electrodes with one electrode (the positive one) in the oxidized state and the other one (the negative) in the neutral state (frequently this state is erroneously called the reduced state, possibly because it is created by reduction of the oxidized state) should provide a cell voltage and be capable of storing energy. Because the electrode potentials will be rather close even in the charged state such a cell would not be very attractive as has been pointed out earlier [139][140][141]. Nevertheless such arrangements are still pursued as critically noticed [78]. A second concept employing different ICPs with sufficiently different redox potentials of the neutral-to-oxidized state transition has been considered [139], in a third one possible only with few ICPs mostly from the thiophene family oxidation and reduction of the same ICP present in both electrodes is used. The CV of a polythiophene prepared by electropolymerization of ethyl-p-(3-thienyl)-benzoate (see inset of figure) displayed in Figure 18 shows a typical example.
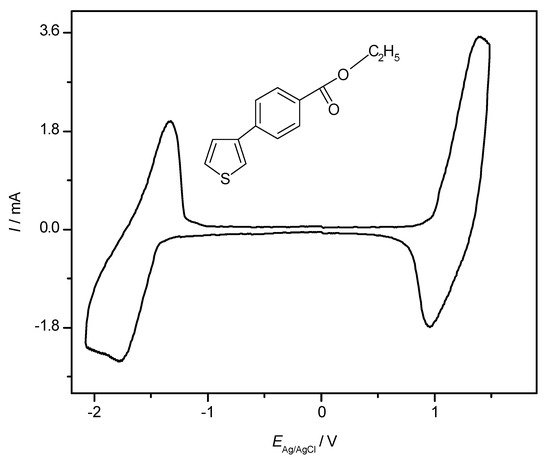
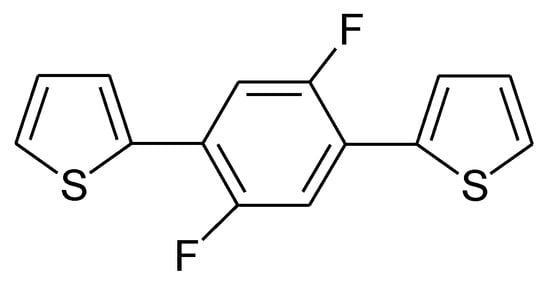
Reduction of substituted PTys can be further supported by suitable substituents like fluorine [147][148][149][150][151] (for an example see Fig. 19) or phenyl units in poly(3-phenyl-thiophene) [140], for further examples see [30], for general considerations [152].
The term all-polymer battery refers to the active masses except for the electrolyte solution. In an all-solid-state battery this concept is developed further by inserting a solid electrolyte or at least a gelled-polymer electrolyte instead of the liquid electrolyte solution commonly used. Some concepts beyond the scope of this report have been presented elsewhere [30].
A frequently encountered drawback of many ICPs is their insolubility in most solvents and their generally poor processeability. Notable exceptions are some polymers prepared from substituted monomers and oligomers having molecular weights (i.e. chain lengths) sufficiently large to show typical properties of an ICP and short enough to enable solubility or at least preparation of stable dispersions like with PEDOT.
Modeling and theoretical tools have been applied to conjugated oligo- and polymers for secondary batteries and to full devices, for an early example see [153].
Nanostructured ICPs in particular with nitrogen as heteroatom have been used as starting material for pyrolytic generation of nanostructured carbon as electrode material or as supporting material. The nanostructure of the starting material frequently serves as template for the obtained carbon material. In addition the nitrogen heteroatoms support high electronic conductance [113].
6. Perspectives
The use of intrinsically conducting polymers in secondary batteries is attractive with respect to availability of the materials, their environmental compatibility and their sustainability. Selection of monomers and ICPs should take into account complexity of their synthesis, and ICPs requiring complex synthesis procedures may be attractive from a scientific point of view but may turn out to be economically disappointing. Stability of the materials in their various functions is still insufficient or at least disappointing in many cases. Accordingly, more extensive lifetime studies under realistic conditions (in terms of minimum cycle number, depth of discharge, currents) are recommended. If possible actual usage settings and operating parameters (like electronic supervision circuits suppressing overcharge and overoxidation) should be considered. A deeper understanding of their behavior in particular with respect to degradation processes based on intensified research yielding insights into options of enhancing stability by avoiding degrading operating conditions might help along this way.
References
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage, VCH-WILEY: Weinheim, Germany 2021.
- Passiniemi, P.; Österholm J.E. Critical aspects of organic polymer batteries. Synth. Met. 1987, 18, 637-644.
- Handbook of Battery Materials, 2nd ed.; (C. Daniel, J.O. Besenhard Ed.) WILEY-VCH: Weinheim, Germany 2011.
- Holze, R. Porphyrinoids as active masses in electrochemical energy storage. In Smart Materials Applications of Porphyrinoids as Functional Materials; Lang, H., Rüffer, T., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2021; Volume 38, pp. 79–91.
- Kondratiev, V.V.; Holze, R. Intrinsically conducting polymers and their combinations with redox-active molecules for rechargeable battery electrodes: an update. Chem. Pap. 2021, 75, 4981-5007.
- Holze, R. Optical and Electrochemical Band Gaps in Mono-, Oligo-, and Polymeric Systems: A Critical Reassessment. Organometallics 2014, 33, 5033-5042.
- Werner, HP; Grauf, W.; von Schütz, J.U.; Wolf, H.C.; Hedberg, H.W.; Kremer, W.; Aumüller, A.; Hünig, S. Conductivity and magnetic properties of the charge-transfer complex from N,N'-Dicyanonaphthoquinonediimine (DCNNI) and tetrathiafulvalene (TTF). Z. Naturforsch. 1989, 44a, 825-832.
- Hünig, S.; Aumüller, A.; Erk, P.; Meixner, H.; von Schütz, J.U. Synthesis and Structure of New Anion Radical Salts from DCNQIs . Synth. Met. 1988, 27, B181-B188.
- Handbook of conducting polymers 2nd ed. (T.A. Skotheim Ed.) Marcel Dekker Inc., New York 1998.
- Conducting Polymer Hybrids (V. Kumar, S. Kalia, H.C. Swart Eds. Ed.), Springer: Cham 2017.
- Conducting polymers, special applications (L. Alcácer Ed.) D. Reidel Publishing Company, Dordrecht 1987.
- Wan, M. Conducting Polymers with Micro and Nanostructures, Springer-Verlag: Heidelberg 2008.
- Inzelt, G. Conducting Polymers - A New Era in Electrochemistry (F. Scholz Ed.) Springer-Verlag: Berlin 2008.
- Holze, R.; Stejskal, J. Recent trends and progress in research into structure and properties of polyaniline and polypyrrole - Topical Issue. Chem. Pap. 2013, 67, 769-770
- Rasmussen, S.C. Early history of conjugated polymers: From their origin to the Handbook of Conducting Polymers. In: Handbook of conducting polymers Vol. 2 (J.R. Reynolds, B.C. Thompson, T.A. Skotheim Ed.) CRC Press: Boca Raton 2019, pp. 1-35.
- Cericola, D.; Kötz, R. Hybridization of rechargeable batteries and electrochemical capacitors: Principles and limits. Electrochim. Acta 2012, 72, 1-17.
- Wu, Y.; Holze, R. Battery and/or Supercapacitor electrode? On the Merger of two Electrochemical Storage Families, submitted for publication to ChemTexts.
- Holze, R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes-An Introduction. Polymers 2020, 12, 1835.
- Holze, R.; Wu, Y.P. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93-107.
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1-12.
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268-285.
- Li, Q.; Horn, M.; Wang, Y.; MacLeod, J.; Motta, N. A review of supercapacitors based on graphene and redox-active organic materials. Materials 2019, 12, 703.
- Liangliang, T.; Chunyang, J. Conducting Polymers as Electrode Materials for Supercapacitors. Prog. Chem. 2010, 22, 1610-1618.
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting Polymer Nanowire Arrays for High Performance Supercapacitors. Small 2014, 10, 14-31.
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A. Recent developments in conducting polymers: applications for electrochemistry. RSC Adv. 2020, 10, 37834-37856.
- Ramya, R.; Sivasubramanian, R.; Sangaranarayanan, M.V. Conducting polymers-based electrochemical supercapacitors-Progress and prospects. Electrochim. Acta 2013, 101, 109-129.
- Han, Y.; Dai, L. Conducting Polymers for Flexible Supercapacitors. Macromol. Chem. Phys. 2019, 220, 1800355.
- Cheng, M.; Meng, Y.N.; Wei, Z.X. Conducting Polymer Nanostructures and their Derivatives for Flexible Supercapacitors. Isr. J. Chem. 2018, 58, 1299-1314.
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978-1987.
- Jia, X.; Ge, Y.; Shao, L.; Wang, C.; Wallace, G.G. Tunable Conducting Polymers: Toward Sustainable and Versatile Batteries. ACS Sust. Chem. Engin. 2019, 7, 14321-14340.
- Zhu, L.; Shen, Y.; Sun, M.; Qian, J.; Cao, Y. Self-doped polypyrrole with ionizable sodium sulfonate as a renewable cathode material for sodium ion batteries. Chem. Commun. 2013, 49, 11370-11372.
- Yuan, M.; Minteer, S.D. Redox polymers in electrochemical systems: From methods of mediation to energy storage. Curr. Opin. Electrochem. 2019, 15, 1-6.
- Oyaizu, K.; Nishide, . Redox-active polymers as an organic energy storage material in: Handbook of conducting polymers 4th ed. (J.R. Reynolds, B.C. Thompson, T.A. Skotheim Ed.) CRC Press: Boca Raton 2019, pp. 587-594.
- Casado N.; Mecerreyes, D. Introduction to Redox Polymers: Classification, Characterization Methods and Main Applications in: Redox Polymers for Energy and Nanomedicine (N. Casado, D. Mecerreyes Eds.) RSC: Cambridge 2020, pp. 1-26
- Chepurnaya, I.A.; Karushev, M.P.; Alekseeva, E.V.; Lukyanov, D.A.; Levin, O.V. Redox-conducting polymers based on metal-salen complexes for energy storage applications. Pure Appl. Chem. 2020, 92, 1239-1258.
- Kim, J.; Lee, J.; You, J.; Park, M.S.; Al Hossain, Md S.; Yamauchi, Y.; Kim, J.H. Conductive polymers for next-generation energy storage systems: recent progress and new functions. Mater. Horiz. 2016, 3, 517-535.
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers Persistent Models and New concepts. Chem. Rev. 2010, 110, 4724-4771.
- Liu, T.; Li, Y. Addressing the Achilles' heel of pseudocapacitive materials: Long-term stability. InfoMat 2020, 2, 807-842.
- Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Design Strategies toward Enhancing the Performance of Organic Electrode Materials in Metal-Ion Batteries. Chem 2018, 4, 2786-2813.
- Espinoza-Acosta, J.L.; Torres-Chavez, P.I.; Olmedo-Martinez, J.L.; Vega-Rios, A.; Flores-Gallardo, S. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422-1438.
- Chaleawlert-umpon, S.; Berthold, T.; Wang, X.; Antonietti, M.; Liedel, C. Kraft Lignin as Electrode Material for Sustainable Electrochemical Energy Storage. Adv. Energy Mater. 2017, 4, 1700698.
- Lahiri, A.; Yang, L.; Höfft, O.; Endres, F. Biodegradable Zn-ion battery with a lignin composite electrode and bio-ionic liquid based electrolyte: Possible: In situ energy generation by lignin electrocatalysis. Mater. Adv. 2021, 2, 2676-2683.
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788.
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y. Hydrolysis lignin-based organic electrode material for primary lithium batteries. J. Solid State Electr. 2013, 17, 2611-2621.
- Gnedenkov, S.V.; Opra, D.P.; Sinebryukhov, S.L.; Tsvetnikov, A.K.; Ustinov, A.Y. Hydrolysis lignin: Electrochemical properties of the organic cathode material for primary lithium battery. J. Ind. Engin. Chem. 2014, 20, 903-910.
- Gnedenkova, S.V.; Opra, D.P.; Zemnukhova, L.A.; Sinebryukhov, S.L.; Kedrinskii, I.A. Electrochemical performance of Klason lignin as a low-cost cathode-active material for primary lithium battery. J. Energy Chem. 2015, 24, 346-352.
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nature Chem. 2015, 7, 19-29.
- Liu, L.; Solin, N.; Inganäs, O. Bio Based Batteries. Adv. Energy Mater. 2021, 11, 2003713.
- Holze, R. From current peaks to waves and capacitive currents-on the origins of capacitor-like electrode behavior. J. Solid State Electr. 2017, 21, 2601-2607.
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751-3769.
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: from the Leyden jar to electric busses. Chemtexts 2016, 2, 13.
- Dubal, D.P.; Wu, Y.; Holze, R. Supercapacitors as fast storage systems for electric energy. Bunsen-Magazin 2015, 17, 216-227.
- Ge, Y.; Xie, X.; Roscher, J.; Holze, R.; Qu, Q. How to measure and report the capacity of electrochemical double layers, supercapacitors, and their electrode materials. J. Solid State Electr. 2020, 24, 3215-3230.
- Scotto, J.; Marmisolle, W.A.; Posadas, D. About the capacitive currents in conducting polymers: the case of polyaniline. J. Solid State Electr. 2019, 23, 1947-1965.
- Lippe, J.; Holze, R. Electrochemical in-situ conductivity and polaron concentration measurements at selected conducting polymers. Synth. Met. 1991, 41-43, 2927-2930.
- Zerbi, G.; Veronelli, M.; Martina, S.; Schlüter, A.D.; Wegner, G. pi-Electron Delocalization in Conformationally Distorted Oligopyrroles and Polypyrrole, Adv. Mater. 1994, 6, 385-388.
- Brandl, V.; Holze, R. Influence of the preparation conditions on the properties of electropolymerised polyaniline. Ber. Bunsenges. Phys. Chem. 1997, 101, 251-256.
- Holze, R.: Experimental Electrochemistry: A Laboratory Textbook, 2nd ed., VCH-Wiley: Weinheim 2019.
- Wu, Y.; Holze, R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem. Energy Technol. 2021, 7, 1-37.
- Opitz, M.; Yue, J.; Wallauer, J.; Smarsly, B.; Roling, B. Mechanisms of charge storage in nanoparticulate TiO2 and Li4Ti5O12 anodes: New insights from scan rate-dependent cyclic voltammetry. Electrochim. Acta 2015, 168, 125-132.
- Bandeira, M.C.E.; Holze, R. Impedance measurements at thin polyaniline films - the influence of film morphology. Microchim. Acta 2006, 156, 125-131.
- Ko, J.S.; Lai, C.H.; Long, J.W.; Rolison, D.R.; Dunn, B.; Weker, J.N. Differentiating Double-Layer, Pseudocapacitance, and Battery-like Mechanisms by Analyzing Impedance Measurements in Three Dimensions. ACS Appl. Mater. Interfaces 2020, 12, 14071-14078.
- Holze, R. Surface and Interface Analysis An Electrochemists Toolbox. Springer: Heidelberg 2009.
- Malinauskas, A. Self-doped polyanilines. J. Power Sources 2004, 126, 214-220.
- Malinauskas, A.; Holze, R. Deposition and characterisation of self-doped sulphoalkylated polyanilines. Electrochim. Acta 1998, 43, 521-531.
- Wang, P.H.; Wang, T.L.; Lin, W.C.; Lin, H.Y.; Lee, M.H.; Yang, C.H. Enhanced Supercapacitor Performance Using Electropolymerization of Self-Doped Polyaniline on Carbon Film. Nanomaterials 2018, 8, 214.
- Ghenaatian, H.R.; Mousavi, M.F.; Rahmanifar, M.S. High performance battery-supercapacitor hybrid energy storage system based on self-doped polyaniline nanofibers. Synth. Met. 2011, 161, 2017-2023.
- Ghenaatian, H.R.; Mousavi, M.F.; Kazemi, S.H.; Shamsipur, M. Electrochemical investigations of self-doped polyaniline nanofibers as a new electroactive material for high performance redox supercapacitor. Synth. Met. 2009, 159, 1717-1722.
- Malinauskas, A.; Bron, M.; Holze, R. Electrochemical and Raman spectroscopic studies of electrosynthesized copolymers and bilayer structures of polyaniline and poly(o-phenylenediamine). Synth. Met. 1998, 92, 127-137.
- Ghenaatian, H.R.; Mousavi, M.F.; Rahmanifar, M.S. High performance hybrid supercapacitor based on two nanostructured conducting polymers: Self-doped polyaniline and polypyrrole nanofibers. Electrochim. Acta 2012, 78, 212-222.
- Bian, L.J.; Luan, F.; Liu, S.S.; Liu, X.X. Self-doped polyaniline on functionalized carbon cloth as electroactive materials for supercapacitor. Electrochim. Acta 2012, 64, 17-22.
- Wang, Y.; Jiang, H.; Zheng, R.; Pan, J.; Niu, J.; Zou, X.; Jia, C. A flexible, electrochromic, rechargeable Zn-ion battery based on actiniae-like self-doped polyaniline cathode. J. Mater. Chem. A 2020, 8, 12799-12809.
- Li, Y.; Wang, H.; Yu, T. Li, J.; Kan, J. Cheap and eco-friendly synthesis of poh aniline and performance of aqueous Zn-polyaniline battery. Int. J. Electrochem. Sci. 2020, 15, 5956-5965.
- Li, X.; Lv, R.; Zou, S.; Na, B.; Liu, P.; Ma, Y.; Liu, H. Polyaniline nanopillars on surface cracked carbon fibers as an ultrahigh-performance cathode for a flexible rechargeable aqueous Zn-ion battery. Compos. Sci. Technol. 2019, 180, 71-77.
- Franco-Gonzalez, J.F.; Pavlopoulou, E.; Stavrinidou, E.; Gabrielsson, R.; Simon, D.T.; Berggren, M.; Zozoulenko, I.V. Morphology of a self-doped conducting oligomer for green energy applications. Nanoscale 2017, 9, 13717-13724.
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985-1996.
- Yin, Z.; Zheng, Q. Controlled Synthesis and Energy Applications of One-Dimensional Conducting Polymer Nanostructures: An Overview. Adv. Energy Mater. 2011, 20, 1-40.
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937-14970.
- Lippe, J.; Holze, R. The anion-specific effect in the overoxidation of polyaniline and polyindolines. J. Electroanal. Chem. 1992, 339, 411-422.
- Goncalves, R.; Pereira, E.C.; Marchesi, L.F. The Overoxidation of poly(3-hexylthiophene) (P3HT) Thin Film: CV and EIS measurements. Int. J. Electrochem. Sci. 2017, 12, 1983-1991.
- Bouabdallaoui, M.; Aouzal, Z.; Ben Jadi, S.; El Jaouhari, A.; Bazzaoui, M.; Lévi, G.; Aubard, J.; Bazzaoui, E.A. X-ray photoelectron and in situ and ex situ resonance Raman spectroscopic investigations of polythiophene overoxidation, J. Solid State Electr. 2017, 21, 3519-3532.
- Láng, G.G.; Ujvári, M.; Vesztergom, S.; Kondratiev, V.; Gubicza, J.; Szekeres, K.J. The Electrochemical Degradation of Poly(3,4-ethylenedioxythiophene) Films Electrodeposited from Aqueous Solutions. Z. Physik. Chem. 2016, 230, 1281-1302.
- Novak, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically Active Polymers for Rechargeable Batteries. Chem. Rev. 1997, 97, 207-281.
- Mike, J.F.; Lutkenhaus, J.L. Recent advances in conjugated polymer energy storage. J. Polym. Sci. B 2013, 51, 468-480.
- Mike, J.F.; Lutkenhaus, J.L. Electrochemically active polymers for electrochemical energy storage: Opportunities and challenges. ACS Macro Lett. 2013, 2, 839-844.
- Xiang, H.; Deng, N.; Zhao, H.; Wang, X.; Wei, L.; Wang, M.; Cheng, B.; Kang, W. A review on electronically conducting polymers for lithium-sulfur battery and lithium-selenium battery: Progress and prospects. J. Energy Chem. 2021, 58, 523-556.
- Eliseeva, S.N.; Shkreba,E.V.; Kamenskii, M.A.; Tolstopjatova, E.G.; Holze, R.; Kondratiev, V.V. Effects of conductive binder on the electrochemical performance of lithium titanate anodes. Solid State Ionics 2019, 333, 18-29.
- Liu, L.L.; Wang, X.J.; Zhu, Y.S.; Hu, C.L.; Wu, Y.P.; Holze, R. Polypyrrole-coated LiV3O8-nanocomposites with good electrochemical performance as anode material for aqueous rechargeable lithium batteries. J. Power Sources 2013, 224, 290-294.
- Miller, J.S. Conducting polymers—Materials of commerce. Adv. Mater. 1993, 5, 671–676. [Google Scholar] [CrossRef]Miller, J.S. Conducting polymers—Materials of commerce. Adv. Mater. 1993, 5, 671–676.
- Miller, J.S. Conducting polymers - Materials of commerce. Adv. Mater. 1993, 5, 671-676.
- Bäuerle, P. Intrinsically conducting polymers-quo vadis? Adv. Mater. 1993, 5, 879-886.
- Nishio, K.; Fujimoto, M.; Yoshinaga, N.; Furukawa, N.; Ando, O.; Ono, H.; Suzuki, T. Characteristics of a lithium secondary battery using chemically-synthesized conductive polymers. J. Power Sources 1991, 34, 153-160.
- Matsunaga, T.; Daifuku, H.; Kawagoe, T. Development of Polyaniline Lithium Secondary Battery. Nippon Kagaku Kaishi 1990, 1990, 1-11.
- Matsunaga, T.; Daifuku, H.; Nakajima, T.; Kawagoe, T. Development of polyaniline-lithium secondary battery. Polym. Adv. Technol. 1990, 1, 33-39.
- Zhou, M.; Li, W.; Gu, T.; Wang, K.; Cheng, S. A sulfonated polyaniline with high density and high rate Na-storage performances as a flexible organic cathode for sodium ion batteries. Chem. Commun. 2015, 51, 14354-14356.
- Liu, Y.; Xie, L.; Zhang, W.; Dai, Z.; Wei, W.; Luo, S.; Chen, X.; Chen, W. Conjugated System of PEDOT:PSS-Induced Self-Doped PANI for Flexible Zinc-Ion Batteries with Enhanced Capacity and Cyclability. ACS Appl. Mater. Interf. 2019, 11, 30943-30952.
- Zhao, R.; Zhu, L.; Cao, Y.; Ai, X.; Yang, H.X. An aniline-nitroaniline copolymer as a high capacity cathode for Na-ion batteries. Electrochem. Commun. 2012, 21, 36-38.
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured conductive polymers for advanced energy storage. Chem. Soc. Rev. 2015, 44, 6684-6696.
- Stejskal, J.; Sapurina, I.; Trchova, M.; Konyushenko, N.; Holler, P. The genesis of polyaniline nanotubes. Polymer 2006, 47, 8253-8262.
- Channu, V.S.R.; Holze, R.; Rambabu, B.; Kalluru, R.R. Synthesis and characterization of PANI nanostructures for supercapacitors and photoluminescence. Iran. Polym. J. 2012, 21, 457-462.
- Simotwo, S.K.; Kalra, V. Polyaniline-based electrodes: recent application in supercapacitors and next generation rechargeable batteries. Curr. Opin. Chem. Eng. 2016, 13, 150-160.
- He, Y.; Wang, X.; Zhang, P.; Huang, H.; Li, X.; Shui, Y.; Chen, B.; Guo, Z. A versatile integrated rechargeable lead dioxide-polyaniline system with energy storage mechanism transformation. Energy 2019, 183, 358-367.
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86-107.
- Banerjee, J.; Dutta, K.; Kader, M.A.; Nayak, S.K. An overview on the recent developments in polyaniline-based supercapacitors. Polym. Adv. Technol. 2019, 30, 1902-1921.
- Li, Z.; Gong, L. Research progress on applications of polyaniline (PANI) for electrochemical energy storage and conversion. Materials 2020, 13, 548.
- Wang, M.; Zhang, J.; Zhang, L.; Li, J.; Wang, W.; Yang, Z.; Zhang, L.; Wang, Y.; Chen, J.; Huang, Y.; Mitlin, D.; Li, X. Graphene-like Vanadium Oxygen Hydrate (VOH) Nanosheets Intercalated and Exfoliated by Polyaniline (PANI) for Aqueous Zinc-Ion Batteries (ZIBs). ACS Appl. Mater. Interfaces 2020, 12, 31564-31574.
- Mohammadi, A.; Inganäs, O.; Lundström, I. Properties of Polypyrrole-Electrolyte-Polypyrrole Cells. J. Electrochem. Soc. 1986, 133, 947-949.
- Münstedt, H.; Köhler, G.; Möhwald, H.; Naegele, D.; Bitthin, R.; Ely, G.; Meissner, E. Rechargeable polypyrrole/lithium cells. Synthet. Met. 1987, 18, 259-264.
- Bittihn, R. Batterien mit elektrisch leitfähigen Polymeren. Kunststoffe 1989, 79, 530-535.
- Bittihn, R.; Ely, G.; Woeffler, F.; Münstedt, H.; Naarmann, H.; Naegele D. Polypyrrole as an electrode material for secondary lithium cells. Makromol. Chem. Macromol. Symp. 1987, 8, 51-59.
- Corradini, A.; Mastragostino, M.; Panero, A.S.; Prosperi, P.; Scrosati, B. Electrochemical stability of electrosynthesized heterocyclic semiconducting polymers as cathode-active materials in advanced batteries. Synth. Met. 1987, 18, 625-630.
- Yamamoto, T.; Zama, M.; Hishinuma, M.; Yamamoto, A. Lithium secondary cells using LiX (X=ClO4, BF4) as electrolyte and poly(2,5-pyrrolylene) and poly(2,5-thienylene) as materials for positive electrodes. J. Appl. Electrochem. 1987, 17, 607-612.
- Dubal, D.; Jagadale, A.; Chodankar, N.R.; Kim, D.H.; Gomez-Romero, P.; Holze, R. Polypyrrole Nanopipes as a Promising Cathode Material for Li-ion Batteries and Li-ion Capacitors: Two-in-One Approach. Energy Technol. 2019, 7, 193-200.
- Dubal, D.P.; Caban-Huertas, Z.; Holze, R.; Gomez-Romero, P. Growth of polypyrrole nanostructures through reactive templates for energy storage applications. Electrochim. Acta 2016, 191, 346-354.
- Bocchetta, P.; Frattini, D.; Tagliente, M.; Selleri, F. Electrochemical deposition of polypyrrole nanostructures for energy applications: A review. Current Nanosci. 2020, 16, 462-477.
- Stejskal, J.; Trchova, M. Conducting polypyrrole nanotubes: a review. Chem. Pap. 2018, 72, 1563-1595.
- Sapurina, I.; Li, Y.; Alekseeva, E.; Bober, P.; Trchova, M.; Moravkova, Z.; Stejskal, J. Polypyrrole nanotubes: The tuning of morphology and conductivity. Polymer 2017, 113, 247-258.
- Kaneto, K.; Inuishi, Y.; Yoshino, K. Characteristics of Polythiophene Battery. Jap. J. Appl. Phys. 1983, 22, L567-L568.
- Kaneto, K.; Inuishi, Y.; Kohno,Y.; Yoshino, K. Electrochemical Preparation of a Metallic Polythiophene Film. J. Chem. Soc. Chem. Commun. 1983, 1983, 382-383
- Kaufman, J.H.; Chung, T.C.; Heeger, A.J.; Wudl, F. Poly(Thiophene): A Stable Polymer Cathode Material. J. Electrochem. Soc. 1984, 131, 2092-2093.
- Tang, J.; Kong, L.; Zhang, J.; Zhan, L.; Zhan, H.; Zhou, Y.; Zhan, C. Solvent-free, oxidatively prepared polythiophene: High specific capacity as a cathode active material for lithium batteries. React. Funct. Polym. 2008, 68, 1408-1413.
- Ryu, K.S.; Lee, Y.; Han, K.S.; Kim, M.G. The electrochemical performance of polythiophene synthesized by chemical method as the polymer battery electrode. Mater. Chem. Phys. 2004, 84, 380-384.
- Aradilla, D.; Estrany, F.; Casellas, F.; Iribarren, J.I.; Alemán, C. All-polythiophene rechargeable batteries. Organic Electronics 2014, 15, 40-46.
- Panero, S.; Prosperi, P.; Kalptse, B.; Scrosati, B. Characteristics of electrochemically synthesized polymer electrodes in lithium cells-II. Polythiophene. Electrochim. Acta 1986, 31, 1597-1600.
- Mastragostino, M.; Arbizzani, C.; Corradini, A.; Marinangeli, A.M. Polythienothiophene As Cathode Active Material - A Comparative-Study with Polythiophene and Polydithienothiophene. Electrochim. Acta 1987, 32, 1589-1593.
- Buttol, P.; Mastragostino, M.; Panero, S.; Scrosati, B. The electrochemical characteristics of a polydithienothiophene electrode in lithium cells. Electrochim. Acta 1986, 31, 783-788.
- Biserni, M.; Marinangeli, A.; Mastragostino, M. Doped Polydithienothiophene: A New Cathode-Active Material. J. Electrochem. Soc. 1985, 132, 1597-1601.
- Zhan, L.; Song, Z.; Zhang, J.; Tang, J.; Zhan, H.; Zhou, Y.; Zhan, C. PEDOT: Cathode active material with high specific capacity in novel electrolyte system. Electrochim. Acta 2008, 53, 8319-8323.
- Winther-Jensen, B.; West, K. Stability of highly conductive poly-3,4-ethylene-dioxythiophene. React. Funct. Polym. 2006, 66, 479-483.
- Conte, S.; Rodríguez-Calero, G.G.; Burkhardt, S.E.; Lowe, M.A.; Abruna, H.D. Designing conducting polymer films for electrochemical energy storage technologies. RSC Adv. 2013, 3, 1957-1964.
- Marriam, I.; Wang, Y.; Tebyetekerwa, M. Polyindole batteries and supercapacitors. Energy Storage Mater. 2020, 33, 336-359.
- Abdelhamid, M.E.; O'Mullane, A.P.; Snook, G.A. Storing energy in plastics: a review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611-11626.
- Naegele, D.; Bittihn, R. Electrically conductive polymers as rechargeable battery electrodes. Solid State Ionics 1988, 28-30, 983-989.
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-Based Organic Batteries. Chem. Rev. 2016, 116, 9438-9484.
- Katz, H.E.; Searson, P.C.; Poehler, T.O. Batteries and charge storage devices based on electronically conducting polymers. J. Mater. Res. 2010, 25, 1561-1574.
- Ramar, A.; Wang, F.M. Advances in polymer electrode materials for alkali metals (lithium, sodium and potassium)-ion rechargeable batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 21832-21855.
- Chiang, C.K. An all-polymeric solid state battery. Polymer 1981, 22, 1454-1456.
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797-828.
- Naoi, K.; Morita, M. Advanced polymers as active materials and electolytes for electrochemical capacitors and hybrid capacitor systems. Interface 2008, 17(1), 44-48.
- Rudge, A.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. A Study of the Electrochemical Properties of Conducting Polymers for Application in Electrochemical Capacitors. Electrochim. Acta 1994, 39, 273-287.
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting Polymers as Active Materials in Electrochemical Capacitors. J. Power Sources 1994, 47, 89-107.
- Al-Anber, M.; Milde, B.; Alhalasah, W.; Lang, H.; Holze, R. Electrochemical and DFT-studies of substituted thiophenes. Electrochim. Acta 2008, 53, 6038-6047.
- Alhalasah, W.; Holze, R. Theoretical treatment of 3-phenylsubstituted thiophenes and their intrinsically conducting polymers. ECS Transactions 2007, 2(23), 45-62.
- Alhalasah, W.; Holze, R. Electrochemical bandgaps of a series of poly-3-p-phenylthiophenes. J. Solid State Electr. 2007, 11, 1605-1612.
- Alhalasah, W.; Holze, R. Electrochemical materials science: calculation vs. experiment as predictive tools in tailoring intrinsically conducting polythiophenes. Microchim. Acta 2006, 156, 133-139.
- Alhalasah, W.; Holze, R. Electrochemical materials science: Tailoring intrinsically conducting polymers, The example: Substituted thiophenes. J. Solid State Electr. 2005, 9, 836-844.
- Gofer, Y.; Killian, J.G.; Sarker, H.; Poehler, T.O.; Searson, P.C. The electrochemistry of fluorine-substituted polyphenylthiophenes for charge storage applications. J. Electroanal.Chem. 1998, 443, 103-115.
- Sarker, H.; Gofer, Y.; Killian, J.G.; Poehler, T.O.; Searson, P.C. Synthesis and characterization of a series of fluorine- substituted phenylene-thienyl polymers for battery applications. Synth. Met. 1998, 97, 1-6.
- Ferraris, J.P.; Eissa, M.M.; Brotherston, I.D.; Loveday, D.C. Performance evaluation of poly 3-(phenylthiophene) derivatives as active materials for electrochemical capacitor applications. Chem. Mater. 1998, 10, 3528-3535.
- Killian, J.G.; Gofer, Y.; Sarker, H.; Poehler, T.O.; Searson, P.C. Electrochemical synthesis and characterization of a series of fluoro-substituted phenylene-2-thienyl polymers. Chem.Mater. 1999, 11, 1075-1082.
- Sarker, H.; Gofer, Y.; Killian, J.G.; Poehler, T.O.; Searson, P.C. Synthesis and characterization of fluoro-substituted polyphenylthiophenes for charge storage applications. Synth. Met. 1997, 88, 179-185.
- Levi, M.D.; Aurbach, D. A short review on the strategy towards development of pi-conjugated polymers with highly reversible p- and n-doping. J. Power Sources 2008, 180, 902-908.
- Passiniemi, P.; Inganäs, O. Modelling of polymer batteries. Solid State Ionics 1989, 34, 225-230.
- Miller, J.S. Conducting polymers—Materials of commerce. Adv. Mater. 1993, 5, 671–676. [Google Scholar] [CrossRef]
