You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Faheem Ahmad.
The green synthesis of NPs is gaining attention owing to its facilitation of the development of alternative, sustainable, safer, less toxic and environment-friendly approaches. Thus, green nanotechnology using plant extract opens up new possibilities for the synthesis of novel nanoparticles with the desirable characteristics required for developing biosensors, biomedicine, cosmetics and nano-biotechnology, and in electrochemical, catalytic, antibacterial, electronics, sensing and other applications.
- biosynthesis
- eco-friendly
- green chemistry
- nanoparticle
- plant extract
- sustainable application
1. Introduction
The nanotechnology sector has proven to be one of the most active research fields [1]. Owing to their broad uses in catalysis, sensing, electronics, photonics and medicines, the synthesis of nanoparticles has gained significant attention in recent decades [2]. Scientists have understood the potential of biological organisms to reduce metal precursors since the nineteenth century, but the mechanisms are still not known. Researchers have drawn attention towards biological methods due to the success of nanoparticle synthesis using natural reduction, capping and stabilizing agents, and avoiding harmful chemicals and high energy consumption [3,4,5]. A wide variety of products (e.g., Quantum dots (Q-dots) of cadmium sulphide, titanium oxide hybrid-based electrochemical biosensors and oxorubicin-loaded heparinized nanoparticles) can be developed through nanotechnology, and applicable to a broad array of scientific fields, including optoelectronics, biosensors, nano-biotechnology, biomedicine and others [6,7,8,9]. Creation, exploitation and synthesis are nanotechnology concepts that typically consider materials smaller than 1 mm in dimension [10]. Many different methods, such as physical, chemical and green (biological) techniques, have been used to synthesize nanoparticles [11,12,13]. The stabilized nanoparticles are formed by reducing ions through reduction (palladium NPs), nucleation (silver NPs) and growth system (silver NPs) [14,15,16]. Green chemistry, which uses chemical principles to reduce or eliminate the use of hazardous substances, has led to considerable reductions in toxic residues, which are harmful to man and the environment.
Green chemistry may be defined as chemical-assisted pollution-prevention strategies employed in specific domains such as green analytical chemistry, ecologically friendly analytical chemistry and clean analytical methodologies [17]. Thus, green synthesis is regarded as a viable approach for nanoparticle synthesis since it is biocompatible, inert and environmentally safe [18].
2. Biosynthesis of Novel Metal Nanoparticles Using Plant Extracts
Nanoparticles with sizes ranging from 1 to 100 nm bind larger particles to atomic or molecular structures [23]. They are synthesized via different approaches, mainly divided into physical and chemical processes (Figure 2). The physical process involves laser ablation, condensation, evaporation, etc., whereas the chemical process involves hydrazine, sodium borohydride, green synthesis, etc. Using plant species to produce nanoparticles has been termed a green technique (Figure 2 and Figure 3) and the most reliable environmentally sustainable approach [24,25]. Nowadays, researchers are attracted towards biological synthesis, including the use of natural reducing, capping and stabilizing agents and without using hazardous, high-cost chemicals and high power consumption [26] (Figure 2 and Figure 3). NPs are extensively utilized in human contact areas (medicine, [27,28] and agriculture, [29,30]), and synthesis methods that do not use harmful compounds are increasingly required.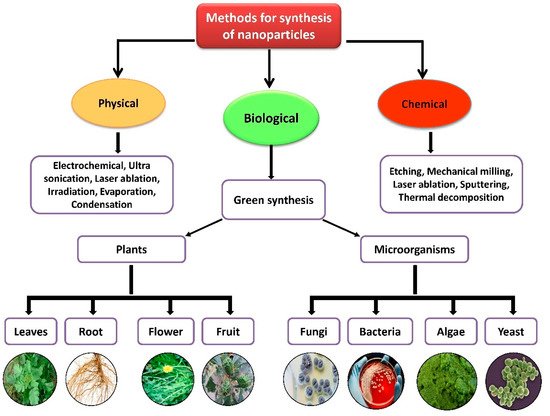
Figure 2.
Different methods of nanoparticle synthesis.
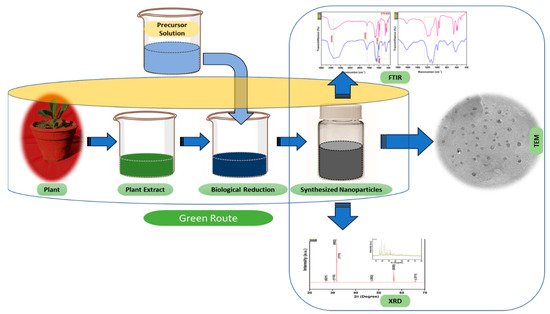
Figure 3.
The schematic diagram for the biosynthesis of nanoparticles (NPs) via a green route using plant extract.
2.1. Mechanism of Nanoparticle Synthesis
Extensive research has been published on the testing and assessing of plants to prepare metallic nanoparticles (Figure 3), but the underlying principle for synthesizing nanomaterials has received comparatively less scientific attention [31,32]. The general tools, steps and materials involved in nanoparticle synthesis include reducing agents, capping agents, solvents, metal salts, nucleation, growth, aggregation, stabilization and characterization (Figure 4). Chemical reduction is commonly used in nanoparticle synthesis. Most methods utilize highly reactive reducing agents such as amino acids, citric acid, aldehydes, flavonoids, NADP reductase, tartaric acids, secondary metabolites, etc. Two researchers reported that the reduction potential of each metal is different and greatly affect the reduction of metals or metal precursors during synthesis. If the positive reduction potential is more, the metal precursor can be reduced at a faster rate. The nucleation and growth phases will be close to equilibrium when the reducing rate is slow [33,34]. In one-step synthesis, the slow reduction rate is also a key factor in the production of Au−Pd core–shell NPs. The finding reported the reduction potentials of PdCl42−/Pd and AuCl4−/Au are 0.59 and 0.99 eV, respectively. As confirmed from the TEM analysis, during reaction the Au particles were synthesized earlier then Pd at different time intervals. This is highly consistent with PdCl42−/Pd and AuCl4−/Au’s redox potential difference, and it is believed that this difference is very important for the development of the core–shell NPs [34]. In the water-soluble components of geranium leaves, Shankar et al. [35] recognized proteins and secondary metabolites. They suggested that terpenoids aid in reducing silver ions, which are then oxidized to carbonyl groups. In a study with tamarind leaf broth, the probability of an acid (tartaric acid) functional group operating as a capping medium and being essential for forming bio-reduced gold nanoparticles was studied by Ankamwar et al. [36]. This study investigated the way that alfalfa roots can absorb silver from agar media in the form of Ag(0) and transmit it to the shooting segment in the identical oxidation number [37]. The synthesized nanoparticles’ general characterization was carried out through scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), ultraviolet–visible spectroscopy (UV–Vis), Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD). Microscopy (SEM and TEM) is used to determine the shape, size and particle aggregation of the desired nanoparticles without any comparison with standard materials [38]. Spectrometric techniques are the most widely used tactic for nanoparticle characterization. EDX is used to confirm the composition and distribution of the nanoparticles through spectrum and element mapping. The UV–Vis spectrometry investigates nanoparticles on the basis of particle aggregation and average particle size [39]. The basic principle of this method is absorption of plasmas by free electrons attached on the surface of nanoparticles. They interact with the electromagnetic field and shift towards higher wavelength values because the size of nanoparticles is directly proportional to higher values of wavelength. Furthermore, FTIR and XRD are applied for the determination of structural characteristics and crystallinity of formed particles.
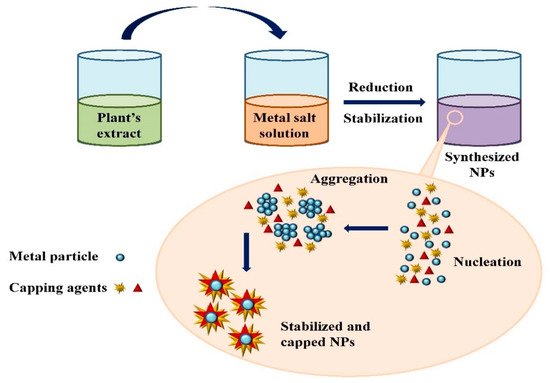

Figure 4.
Mechanism of nanoparticle synthesis using phytoextracts.
The information on the production of various metallic NPs such as silver, gold, zinc, palladium and titanium using various plant extracts is summarized here.
2.2. Silver Nanoparticles
Silver nanoparticles (AgNPs) are commonly utilized nanoparticles and have attracted much study interest due to their distinctive properties. They are widely used in emerging biomedical and industrial applications [40]. AgNPs exhibit completely different characteristics from bulk materials derived from the same material due to their elevated surface/volume ratio [41]. In recent times, the synthesis of silver NPs by bio-organisms containing phytochemical agents has become an important goal for workers. Various unique secondary metabolites derived from plant extracts such as sugars, alkaloids, phenolic acids, flavonoids and terpenoids are responsible for bio-reducing ionic silver metal into nanoparticles [25,42,43].
Biosynthesis of AgNPs by Tribulus terrestris [44] and Astragalus tribuloides Delile [45] has already been reported. Spherical silver nanoparticles of size 2–6 nm were obtained from Cycas leaf [46]. For the synthesis of AgNPs, the affinity of Curcuma longa bark and powder extracts was determined. It was found that bark extract could produce more AgNPs than powder extract [47]. Kumar and Yadav [48] investigated Lonicera japonica plant leaf extract to develop silver and gold nanostructures. The particles obtained were different in size and shape; AgNPs were spherical to plate-like poly-shaped, and their size was 36–72 nm. Banerjee and Narendhirakannan [49] utilized seed extract of Syzygium cumuni to form crystalline silver nanoparticles. There is considerable data available on how to make silver nanoparticles from the latex of the Plumeria rubra plant [50]. Ponarulselvam et al. [51] evaluated Catharanthus roseus to produce silver nanoparticles because of the presence of vincristine and vinblastin. Sathishkumar et al. [52] prepared silver nanoparticles using Cinnamomum zeylanicum bark extract and powdered bark extract and studied the variations in the biogenic nanoparticles.
AgNPs were synthesized with a 58–458 nm range in size from the leaf extract of Mukia maderaspatana [53]. Pedalium murex was also reported to synthesize AgNPs by Anandalakshmi et al. [54]. The TEM micrographs revealed that the produced AgNPs were circular with a mean value of 50 nm. Raju et al. [55] utilized living peanut plants to synthesize AgNPs. The TEM examination showed that the biosynthesized AgNPs were of different shapes (spherical, hexagonal, triangular, square and rod-shaped) and sizes. Most of the formed AgNPs were spherical and 56 nm in average size. The EDX technique confirmed that the formed NPs were of silver. Some reports on plant-assisted synthesis of silver nanoparticles are enlisted below in Table 1.
AuNPs were made from the aqueous suspension of Azadirachta indica [86]. When the A. indica extract was mixed with Au(III) solution, the nanoparticle formation commenced. Kasthuri et al. [87] constructed gold nanoparticles with triangular and hexagonal shapes from HAuCl4 solution and a diluted extract possessing phyllanthin (derived from Phyllanthus amarus). Aromal and Philip [88] synthesized AuNPs using Benincasa hispida seed extract as either a reducing or capping agent. Carboxylic groups (COOH) found in the plant extract change to COO- during the reduction process. The protein’s COOH group works as a surfactant, adhering to the surface of the AuNPs and then stabilizing AuNPs via electrostatic stabilization. The synthesized AuNPs were observed to have a crystalline nature and were 10–30 nm in size. Some reports on the plant-assisted synthesis of gold nanoparticles are listed below in Table 2.
Table 2. Plant-assisted synthesis of gold nanoparticles.
Plant-assisted synthesis of gold nanoparticles.
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Parkia biglobosa | Leaves | 1–35 | Truncated, pentagonal, spherical, triangular | |
| Spherical | [ | 58] | ||
| [ | ||||
| 70 | ||||
| ] | ||||
| Cuminum cyminum | ||||
| Seeds | ||||
| 1–10 | ||||
| Spherical | ||||
| [ | ||||
| 102 | ||||
| ] | ||||
| Sorbus aucuparia | Leaf extract | 16–18 | Spherical, triangular, hexagonal | [103] |
2.4. Zinc Nanoparticles
Zinc oxide (ZnO) is an inorganic metal oxide with a vast range of nanostructures. Zinc nanoparticles (ZnNPs) have gained considerable attention due to their low cost, large surface area, white appearance, UV-filtering, antifungal, antibacterial and photochemical properties, and high catalytic activity [104,105]. There are several reports of ZnO nanoparticle synthesis using various plant extracts [106,107,108,109]. Plant extracts contain some phytochemicals (i.e., polyphenols, saponins, terpenoids) that act as reducing and stabilizing agents in the reaction system. Phytochemicals are synthesized in the plant parts, including root, stem, leaf, fruit and seed. These phytochemicals lower the metal’s valence to zero, then calcinate it to add oxide. Additionally, zinc ions interact with the polyphenols in the plant extract to form a complex. After that, zinc hydroxide (Zn(OH)2) is formed via hydrolysis, and then ZnO nanoparticles are synthesized after complex calculations [110].
During the literature survey, it was observed that members of the Fabaceae, Rutaceae, Apocynaceae, Solanaceae and Lamiaceae families are most commonly employed for the production of ZnNPs (Table 3). Plants from the family Lamiaceae, such as Anisochilus carnosus, Plectranthus amboinicus and Vitex negundo were used to produce ZnO nanoparticles of different sizes and shapes, including hexagonal, spherical, quasi-spherical and rod-shaped particles. The findings indicated that the particle sizes decrease when plant extract concentration increases [111,112]. All experiments displayed nanoparticles in the same size range with spherical and hexagonal disc shapes, which XRD and TEM analysis characterized. Singh et al. [113] synthesized ZnO NPs using Calotropis procera latex that were spherical and 5 nm to 40 nm in size. Ramesh et al. [114] used the floral extract of Cassia auriculata to react with Zn(NO3)2 solution resulting in the development of ZnNPs with a particle size ranging from 110 nm to 280 nm. Some reports on the plant-assisted synthesis of zinc nanoparticles are listed below in Table 3.
168]. The average particle size of synthesized NPs was 10 nm at 90°C, which was revealed from the UV spectrum of the colloidal solution. Palladium NPs were generated with the bark extract of Cinnamomum zeylanicum and PdCl2 solution at 30 °C [169]. Khan et al. [170] carried out the plant-assisted synthesis of PdNPs from the extract of Pulicaria glutinosa and PdCl2. After stirring the mixture of PdCl2 + extract at 90 °C for 2 h, the colour changed from pale yellow to dark brown, indicating the production of PdNPs, validated by UV–visible spectroscopy. A TEM monograph revealed the particle size of the obtained Pd nanoparticles ranged between 20 nm and 25 nm. The particle size of the synthesized NPs was found to be between 10 nm and 50 nm. The biosynthesis of Pd nanoparticles from the leafy solution of Glycine max has been reported [171]. The shape of the particles was found to be uniformly spherical with a 15 nm diameter, which was confirmed by TEM micrograph. Jia et al. [172] performed the synthesis of Pd nanoparticles utilizing Gardenia jasminoides extract containing various antioxidants such as geniposide, crocins, crocetin and chlorogenic acid, which reduce and stabilize the nanoparticles. There are some reports on plant-assisted synthesis of palladium nanoparticles listed below in Table 5.
Table 5. Plant-assisted synthesis of palladium nanoparticles.
Plant-assisted synthesis of palladium nanoparticles.
| Plant Name | Parts Used | Size (nm) | Shapes | Reference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peganum harmala | Seed | 22.5 ± 5.7 | Spherical] | [ | Curcuma pseudomontana | Rhizome | 20 | Spherical | [89 | |||||||
| 173 | ] | Cayratia pedata] | ||||||||||||||
| Leaves | 52.24 | Spherical | [ | |||||||||||||
| Coleus amboinicus | Leaves | 40–50 | Spherical | [174 | Lawsonia inermis | Leaves | 20 | Spherical | [90] | |||||||
| 115 | ] | Caesalpinia pulcherrima | ||||||||||||||
| ] | Euphorbia hirta | Leaves | Gum ghatti20–50 | Spherical | [116] | 4.8 ± 1.6 | Leaves | 9 | Spherical | [ | Cinnamon59] | |||||
| Spherical | Bark | 35 | Spherical | [91] | ||||||||||||
| [ | 175 | ] | Eucalyptus globules | Leaves | 52–70 | Spherical, elongated | [108] | |||||||||
| Filicium decipiens | Leaves | 2–22 | Spherical | [176] | Carya illinoinensis | Leaves | 12–30 | Spherical | Croton Caudatus Geisel | Leaves[ | 20Spherical60] | |||||
| Spherical | [ | 13] | ||||||||||||||
| Tecoma castanifolia | Leaves | 70–75 | Spherical | [117] | [151] | |||||||||||
| Cinnamomum camphora | Leaves | 3.2–6 | Mentha piperita | Leaves extract | 35 | Spherical | [61] | |||||||||
| Tamarind | Leaves | 20–40 | Triangle | |||||||||||||
| Multiple | [ | 177] | Zingiber officinale | Root[ | 30–50 | Psidium guajava | Leaves | 32.58 | Spherical | [152] | ||||||
| Anogeissus latifolia | 36 | ] | ||||||||||||||
| Pulicariaglutinosa | Spherical | [118] | Leaves | Jatropha curcas | Latex | 10–20 | Face-centered cubic | Aloe vera[62] | ||||||||
| 3–5 | Spherical | [170 | Plant extract | 50/350 | Crystalline | [92 | ||||||||||
| ] | Azadirachta indica | ] | ||||||||||||||
| Leaves | 50 | Spindle shaped | [119] | Nyctanthes arbor-tristis | Leaves | 100–150, 100–200 | Cubic, crystalline, Spherical | [153] | Acalypha indica | Leaves extract | 20–30 | Spherical | [63] | |||
| Mentha, Ocimum, Eucalyptus | ||||||||||||||||
| Musa paradisica | Peeled banana | 50 | Crystalline | [178] | Leaves | 3–16 | Spherical | [93] | ||||||||
| Catharanthus roseus | Leaves | 23–57 | Calotropis gigantea | Flower | 10–52Spherical | [120] | Crystalline, Spherical oval | [ | ||||||||
| Cinnamom zeylanicum | Bark | 154 | 15–20 | Crystalline | [169] | Hibiscus rosa sinensis | Leaves | 14 | Spherical/prism | [ | Canna indica, Quisqualis indica64] | |||||
| Leaves and flower | 30–130 | Polymorphic/stable | [94] | |||||||||||||
| Solanum nigrum | ||||||||||||||||
| Catharanthus roseusLeaves | Leaves20–30 | 38Hexagonal | Spherical[121] | [179] | Cycas | Leaves | 2–6 | Spherical | [46] | |||||||
| Murraya koenigii | Leaves | 20 | ||||||||||||||
| Olea europea | Spherical | Leaves[95] | ||||||||||||||
| 18–30 | Crystalline | [122] | Ceratonia siliqua | Leaves extract | 5–40 | Aegle marmelosSpherical | Leaves[ | 4–10 | Spherical | |||||||
| Azadirachta indica | Leaves | [ | 256584] | |||||||||||||
| Crystalline | ] | |||||||||||||||
| [ | 123 | ] | Ocimum tenuiflorum | Leaves | 11–25 | Hexagonal | [127] | |||||||||
| ] | Sargassum muticum | Leaves | 30–57 | Hexagonal | [128] | |||||||||||
| Calotropis gigantea | Leaves | |||||||||||||||
| Salvia officinalis | Leaves | 15–20 | Spherical | [140] | ||||||||||||
| Solanum trilobatum | Leaves | 70 | Spherical, oval | [ | ||||||||||||
| Curcuma longa | 155 | ] | ||||||||||||||
| Tuber | 10–15 | Spherical | [180] | Azadirachta indica | Leaves | 124 | Spherical | [156] | Suaeda monoica | Leaves | Rosa hybrid31 | Spherical | Rose petals | 10 | Cubic | [96 |
| Nyctanthes arbor-tristis | ] | |||||||||||||||
| Flowers | 12–32 | Crystalline[66] | ||||||||||||||
| Catharanthtus roseus | Leaves | 35–55 | Cubical | [51] | ||||||||||||
| [ | Terminalia chebula | Plant extract | 6–60 | Anisotropic | [97] | |||||||||||
| 124 | ] | Ocimum sanctum | ||||||||||||||
| Hibiscus rosa-sinensis | Leaves | 30–35 | Crystal, spongy | [125] | Leaves extract | 10–20 | Spherical | [ | Momordica charantia67] | |||||||
| Fruit | 30–40 | Cubical | [98] | |||||||||||||
| Ruta graveolens | Stem | 28 | Spherical | |||||||||||||
| Glycine max | Leaves | 15 | Spherical | [Annona squamosal | Leaves | 40–60 | Spherical | [157] | ||||||||
| 171 | ] | Jatropha curcas, citrus aurantium | Leaves | 25–50 | Spherical | [158] | [106] | Ocimum tenuiflorum | ||||||||
| Jatropha curcas | Latex | 25–50 | Spherical, uneven | [159] | Leaves | 25–40 | Spherical | Phyllanthus amarus | Leaves[ | 65–9968] | ||||||
| Cubic | [ | 99] | ||||||||||||||
| Aloe vera | ||||||||||||||||
| Euphorbia prostrataLeaves | Leaves22.18 | 81–84Hexagonal | Spherical[126] | [160] | Ginkgo biloba | Mangifera indicaLeaves | 15–500 | Cubic | [ | Leaves69] | ||||||
| 17–20 | Spherical | [100] | Tanacetum vulgare | |||||||||||||
| Citrus sinensis | Fruit peel | 19 | Tetragonal | [161] | Fruit | 16 | Spherical | [70] | ||||||||
| Stevia rebaudiana | Cassia auriculataLeaves | 8–20 | Octahedral | [101] | Leaves | 38 | Spherical | Argemone mexicana | Leaves extract | 30 | Spherical, hexagonal | [71] | ||||
| [ | 162 | ] | Nyctanthes arbortristis | Flower extract | 19.8 | Spherical, hexagonal | [83] | 1.5–8.5 | Spherical | [107 | ||||||
| Ocimum basilicum | ] | Sesuvium portulacastrum | Callus extract | 5–20 | Spherical | [72] | ||||||||||
| Trigonella foneum-graecum | ||||||||||||||||
| Leaves | 50 | Hexagonal | [163] | Leaves | Beta vulgaris15–25 | RootSpherical | 52–76[79 | Hexagonal] | [129] | Syzygium cumini | Leaves and seed | 29–92 | Spherical | [49,73] | ||
| Curcuma longa | Root | 20–80 | Hexagonal | [130] | Cinnamomum camphora | Sun dried leaves | 3.2–20 | Cubic hexagonal crystalline | [74] | |||||||
| Melia azedarach | ||||||||||||||||
| Tanacetum vulgare | Fruit | 11 | Triangular | |||||||||||||
| Nephelium lappaceum | Peel | 20 | Spherical | [131] | Leaves | 78 | Spherical | [75] | ||||||||
| Artocarpus gomezianus | Fruit | 50 | Spherical | [132] | Rhododedendron dauricam | Flower extract | 25–40 | Spherical | [76] | |||||||
| Senna auriculata | Leaves | 2 | Spherical | [133] | Lippia citriodora | Leaves extract | 15–30 | Crystalline | [77] | |||||||
| Brassica oleraceae | Leaves | 1–100 | Spherical and sheet shaped | [134 | Tribulus terrestris | Fruit | 16–28 | Spherical | [44] | |||||||
| Citrullusm colocynthis | Leaves | 31 | Spherical | [78] |
2.3. Gold Nanoparticles
Gold nanoparticles (AuNPs) are the most appealing new metal NPs due to their remarkable uses in catalysis, gene expression, nonlinear optics, nanoelectronics and disease diagnostics fields [79]. Gold nanoparticles made using either phytochemicals or other extract constituents are stable for a limited period [80]. According to Sharma et al. [81], tea leaf extract can be employed in gold NP preparation. Suman et al. [82] synthesize gold NPs of size range 8–17 nm from the root extracts of Morinda citrifolia at ambient temperature. The biogenic production of gold nanoparticles exploiting Nyctanthes arbortristis alcoholic extract led to the creation of spherical-shaped nanostructures of size 19.8 ± 5.0 nm [83]. The synthesis of AuNPs was reported with Bael (Aegle marmelos) leaves and the particles obtained were round and 4–10 nm in size [84].
Lee et al. [38] performed the synthesis of AuNPs from the peel aqueous extract of Garcinia mangostana. The aqueous solution of gold in contact with G. mangostana extract was reduced to gold metal ions and synthesized AuNPs. The FTIR results suggested that the reducing agent found in the aqueous solution of G. mangostana is strongly associated with anthocyanins, benzophenones, flavonoids and phenols. The synthesized AuNPs were spherical with a size range of 32.96 ± 5.25 nm that was analyzed by TEM. Rodríguez-León et al. [85] synthesized AuNPs from the bark extract of Mimosa tenuiflora at different metallic (acting as precursor) concentrations.
2.5. Titanium Nanoparticles
Titanium dioxide nanoparticles (TiNPs) have drawn great attention because of their appropriate electrical band structure, high specific surface area and quantum efficacy, stability, and chemical innerness [139]. TiNPs have a wide applicability in lowering the toxicity of synthetic dyes [140] and pharmaceutical medicines [141], wastewater treatment [142], etc. The synthesis of TiO2 nanoparticles on a wide scale using biological methods has stimulated the interest of researchers due to its low cost, environmental friendliness and reproducibility. Nowadays, there are many reports on the biosynthesis of TiO2 nanoparticles by using microbes (such as bacteria and fungi), algae, plant parts and enzymes. The aqueous extract of Eclipta prostrata produce nanoparticles with a spherical shape and sizes ranging from 36 nm to 68 nm, confirmed by XRD and TEM analysis [143]. Subhashini and Nachiyar [144] used the leaf extract of Albizia saman for the production of titanium NPs via a green route. The aqueous TiO2 solution was added dropwise into the leaf extract with stirring at 50 °C resulting in the formation of anatase crystals of TiO2 nanoparticles. The synthesized TiO2 nanoparticles were found to be 41 nm in size and confirmed by XRD analysis. Jalill et al. [145] synthesized the anatase form of TiO2 nanoparticles by using the plant extract of Curcuma longa (because of its terpenoid and flavonoid contents). The nanoparticles that were developed were identified by the techniques of XRD, FTIR, SEM and EDX that revealed the aggregated, circular structure and a particle size of 160–220 nm. TiNPs were synthesized by the utilization of herbal extract (as a bio-reductant) of Echinacea purpurea [146]. The particle size of the synthesized TiO2 nanoparticles was found to be in the 120 nm range. The leaf extract of Psidium guajava includes alcohol and primary and aromatic amines, which aid in producing TiO2 nanoparticles. Some reports on the plant-assisted synthesis of titanium nanoparticles are listed below in Table 4.
Table 4. Plant-assisted synthesis of titanium nanoparticles.
Plant-assisted synthesis of titanium nanoparticles.
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Ledebouria revoluta | Bulb | 47 | Tetragonal | [147] |
| Pouteria campechiana | Leaves | 73–140 | Spherical | [148] |
| Syzygium cumini | Leaves | 22 | Spherical round | [149] |
| Mentha arvensis | Leaves | 20–70 | Spherical | [150] |
| Azadirachta indica | Leaves | 15–50 | ||
| Hibiscus-rosa-sinensis | ||||
| Petals | ||||
| 7–24 | ||||
| Spherical | [ | 12 | ] | |
| Erythrina variegates | Leaves | 39 | Crystalline, spherical | [164] |
2.6. Palladium Nanoparticles
The major studies of most researchers were focused on the biological synthesis of palladium nanoparticles (PdNPs) via plant materials because it is cost-effective, sustainable, and human- and eco-friendly. Plant extracts contain a number of primary and secondary metabolites that transform metal (Pd) salts to PdNPs. Siddiqi and Husen [165] reported that the shape, size and stability of PdNPs depends on concentrations of plant extract, pH, temperature and incubation time. Plant sources including the extracts of leaves, flowers, seeds, fruits, peels and roots were extensively utilized to synthesize Pd nanoparticles.
Gurunathan et al. [166] synthesized Pd nanoparticles from a plant extract of Evolvulus alsinoides. This plant extract has various natural antioxidants, including alkaloids, flavonoids, saponins, tannin, steroids and phenol, which work as reducing and capping tools to synthesize Pd nanoparticles. Nasrollahzadeh et al. [167] used the leaf extract of Hippophae rhamnoides to synthesize PdNPs because the leaf extract has polyphenols that play an important role as reducing and capping agents for nanostructure development. The formed NPs were found to be spherically shaped and ranging from 2.5 nm to 14 nm, which was confirmed by TEM. Pd nanoparticles have been synthesized from the root extract of Salvadora persica, which contains polyphenols that act as reductant and stabilizing agents [
