Electrochemical behaviors, advantages, and disadvantages of existing electrospun nanofiber anode materials were thoroughly reviewed by classifying into four groups according to the lithium storage principles.
- Li-ion battery anode
- electrospun nanofiber
- rechargeable battery
1. Introduction
Lithium-ion batteries are a portable power source with a high energy density and stable electrochemistry that have changed our daily lives. Thanks to technological developments in areas such as smartphones and electric vehicles, there is an increased demand for high energy density and fast-charging lithium-ion batteries that can provide greater power capacity. Recent battery fires and explosions have also led to a desire to improve the safety of energy storage systems [1][2][1,2]. The design of a novel negative electrode material can address the energy density, safety, and rate performance issues of conventional graphite electrodes that cause unsatisfactory electrochemical performances such as low theoretical capacity (372 mAh/g) [3], irreversible electrolyte and lithium consumption based on solid electrolyte interphase (SEI) formation [4], slow lithium intercalation [5], and dendrite formation during fast charging [6]. Resolving these issues has been the focus of publications on novel lithium-ion battery anodes.
Despite positive results seen in the literature, conventional lithium-ion batteries still use graphite anodes. The two main factors preventing most novel technologies from being implemented in commercial lithium-ion batteries are (i) a need to reduce battery prices rapidly (targeting $125/kWh by 2022) [7] [7] and (ii) a large number of specification requirements for commercial battery anode materials such as areal capacity (4 mAh/cm2) [8], electrode density (1.6 g/cm3) [9], electrode volume change (15%) [10], initial coulombic efficiency (90% to 95%) [11], and various target cycling and rate performance specifications dependent on purpose. Implementing major existing anode material research innovations into a commercial lithium-ion battery and designing novel anode materials and structures should be simultaneously pursued to meet the future demands for high energy density, high safety, and fast-charging lithium-ion batteries.
Electrospinning has been identified as the most promising route for designing novel anode materials and structures, owing to the simple process setup and wide variety of electrospinnable materials. The electrospinning process can encourage the implementation of existing anode material research based on the process being able to mass-produce anodes [12][13][14][12,13,14]. Although nanofiber anode material research has mainly focused on developing carbon-, silicon-, and tin-based materials to replace graphite anodes, there have been numerous publications on a wide variety of anode materials thanks to the merits of the electrospinning process. Here, examples to design the advanced anode materials based on the electrospun nanofibers are presented. Heteroatoms and pores were employed to increase the specific capacity of carbon anode [15][16][15,16]. Carbon composited and nanostructured metal and metal oxide anode materials were designed to improve cycling and rate performances [17][18][17,18]. Lithiophilic nanofiber was fabricated to enhance the reversibility of lithium plating and stripping [19]. It is necessary to review previous research into electrospun nanofiber-based anode materials to establish better strategies for implementing nanofiber anode materials in commercial lithium-ion batteries and designing novel nanofiber anode materials for next-generation batteries.
There have been two types of typical review articles on the electrospun nanofiber anode materials: (i) focusing on specific materials and structures (e.g., reviews of carbon, silicon, tin, and their composite nanofibers and nanostructures for lithium rechargeable batteries [20][21][22][23][24] [20,21,22,23,24] and hollow, porous, and hierarchical structured nanofibers for energy applications [25][26][27][25,26,27]) and (ii) providing a broad overview of recent research and developments [28][29][30][31][28,29,30,31]. Regardless of the review types, the electrospun nanofiber anode materials in the previous review articles were mostly categorized into the material classes, for example, carbon, metals, and metal oxides, and so on [28][29][28,29]. The electrochemical behaviors of the anode materials are, however, not simply classified into the material classes as follows: TiO2 stores lithium by insertion, FeOx is lithiated by conversion reaction, and SnOx electrochemically mainly reacts with the lithium by alloying mechanism. In addition, some anode materials have multiple lithium storage mechanisms. As such, it is important to classify the anode materials into the categories of the main lithium storage principle for better understanding of the anode materials. This review focuses on the lithium storage principles and accordingly categorizes the electrospun nanofiber anode materials into the principles. This review aimed to provide inspiration to those working to improve conventional lithium-ion battery performances and pioneering novel routes to next-generation battery success.
2. Basic Lithium Storage Principles
It is crucial to understand the various mechanisms, advantages, and disadvantages of lithium storage in each anode material when designing high performance anodes. Figure 1a shows the anode materials of lithium rechargeable batteries categorized into four main groups based on electrochemical reactions. The first group is that of insertion (or intercalation) anode materials. Lithium-ions are reversibly inserted into and extracted from the periodic microstructure with little or no microstructural change resulting from the insertion/extraction (or intercalation/ deintercalation) mechanism. Representative insertion anode materials such as graphite and Li4Ti5O12 have been successfully commercialized based on their common strengths (e.g., small volume change during the electrochemical reaction and long lifespan based on the structural stability). However, low theoretical specific capacities (e.g., graphite (372 mAh/g) [32] [32] and Li4Ti5O12 (175 mAh/g) [33]), which are the major drawback of insertion materials, have encouraged the development of alternative anode materials such as conversion and alloying anode materials. The second group is that of conversion reaction-based anode materials. These materials store lithium by altering the ionic bonding from a metallic cation and anion to a lithium-ion and anion in ionic compounds such as metal oxides, metal sulfides, and metal selenides. Microstructural changes that occur to the conversion anode materials during the electrochemical reactions separate the materials into small grains consisting of metal and lithium-anion complexes, which provide higher specific capacities in comparison with the insertion anode materials. However, poor kinetics, large volume changes, and the large redox potential hysteresis prevent commercialization of conversion anode materials. The third group is that of alloying anode materials that provide an increase in specific capacity. This is achieved when the lithium atoms form an alloy with the host metallic phase by breaking the inter-atomic bonds of the host material. However, the large volume changes driven by the insertion and extraction of the large amount of lithium cause pulverization, solid electrolyte interphase (SEI) growth, and electrical contact loss. The fourth group is that of plating anode materials. A vast amount of lithium can be stored by the plating mechanism as the lithium is stored in a free volume. The lithium-ions are deposited on the inactive lithium surface by electrochemical reduction and the lithium atoms are stripped from the surface by electrochemical oxidation. However, critical issues such as dendrite growth, severe SEI growth, and dead lithium formation need to be addressed before plating anode materials can be incorporated into commercial lithium rechargeable batteries.
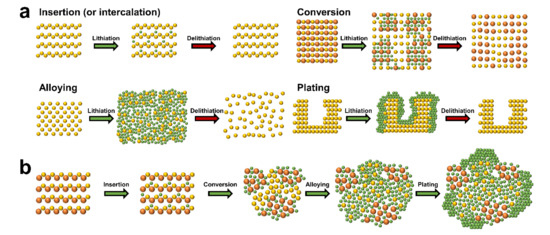
Figure 1.
a
b
The electrochemical behaviors of single element anode materials, such as graphite and crystalline silicon, can be mostly understood from the lithiation mechanisms. For example, lithium storage in artificial graphite is achieved using insertion and plating mechanisms [34]. However, the lithiation behaviors of ceramic anode materials consisting of multiple elements are very complex because of the multiple electrochemical reactions. For instance, tin oxide (SnO2) is lithiated via insertion (SnO2 + xLi+ + xe− ↔ LixSnO2), irreversible conversion (SnO2 + 4Li+ + 4e− → Sn + 2Li2O), and alloying (Sn + xLi+ + xe− ↔ LixSn (0 ≤ x ≤ 4.4)) [35]. When focusing on the microstructural change of specific regions during lithiation, the lithiation mechanism follows a sequential order. The schematic showing the microstructural changes of a virtual ceramic material is shown in Figure 1b to show the sequential order of lithiation: (i) a small amount of lithium atoms stored in the lattice from insertion (or intercalation), (ii) the lithium-ions are bonded to the anions by altering the reduced metallic cations through conversion, (iii) the lithium atoms form an alloy with the fully reduced metallic atoms via alloying, and (iv) the lithium-ions are reduced and deposited on the electrically conductive surface of the ceramic material owing to the fully lithiated microstructure being inactive.
3. Plating/Stripping-Based Storage Materials
Lithium metal with a high specific capacity (3860 mAh/g) and the lowest working potential of the materials discussed (−3.04 V vs. the standard hydrogen electrode (SHE)) has recently been revisited as a highly desirable anode material when pursuing huge energy densities (e.g., 3505 Wh/kg for Li–O2 batteries and 2600 Wh/kg for Li–S batteries) [36][37]. The large volume change observed during the electrochemical reaction and dendrite growth are the primary issues of the lithium metal anode. The dendrite growth causes secondary issues such as continuous SEI formation, dead lithium formation, and electrolyte depletion. Moreover, the dendrite growth has also been well-known as an origin of battery fires, caused by short-circuiting and subsequent thermal runaway. Therefore, suppressing the volume change and dendrite formation is the most important task in lithium metal battery development. Electrospun nanofiber mats are attractive lithium plating/stripping anodes, as their porous structures minimize the volume change and the large specific area of the electrospun mats reduces the current densities. Thus, current research has been focused on improving high performance lithium metal anodes via introducing lithophilic surfaces and regulating lithium deposition behavior.
batteries and 2600 Wh/kg for Li–S batteries) [243,244]. The large volume change observed during the electrochemical reaction and dendrite growth are the primary issues of the lithium metal anode. The dendrite growth causes secondary issues such as continuous SEI formation, dead lithium formation, and electrolyte depletion. Moreover, the dendrite growth has also been well-known as an origin of battery fires, caused by short-circuiting and subsequent thermal runaway. Therefore, suppressing the volume change and dendrite formation is the most important task in lithium metal battery development. Electrospun nanofiber mats are attractive lithium plating/stripping anodes, as their porous structures minimize the volume change and the large specific area of the electrospun mats reduces the current densities. Thus, current research has been focused on improving high performance lithium metal anodes via introducing lithophilic surfaces and regulating lithium deposition behavior. The thickness of an anode can be changed by tens of microns when using a lithium metal anode; a deposition of bare lithium of 1 mAh/cm2 corresponds to a lithium thickness of 4.85 μm [38]. The scaffold or host structure is necessary to reduce the volume change of the lithium metal anode cell on lithiation. The thickness of the nanofiber mat is controlled by the processing time and, in simple terms, a thicker mat can increase the lithium uptake capacity. A lithium/scaffold composite can be easily formed by infusing molten lithium metal into the scaffold, as the melting temperature of lithium is 180 °C [39]. The issue is contact between lithium and the nanofiber mat, and lithophilic nanofiber surface formation has been used to attempt to make the contact intimate (
corresponds to a lithium thickness of 4.85 μm [245]. The scaffold or host structure is necessary to reduce the volume change of the lithium metal anode cell on lithiation. The thickness of the nanofiber mat is controlled by the processing time and, in simple terms, a thicker mat can increase the lithium uptake capacity. A lithium/scaffold composite can be easily formed by infusing molten lithium metal into the scaffold, as the melting temperature of lithium is 180 °C [246]. The issue is contact between lithium and the nanofiber mat, and lithophilic nanofiber surface formation has been used to attempt to make the contact intimate (Figure 2). Zinc oxide was coated on the polyimide (PI) surface using atomic layer deposition to render the matrix wet with molten lithium, as shown in
2). Zinc oxide was coated on the polyimide (PI) surface using atomic layer deposition to render the matrix wet with molten lithium, as shown in a. The composite electrode exhibited little volume change during lithium plating and stripping and had a lower overpotential compared with lithium at all current densities. Electrochemically active carbon nanofibers were also used as the host for the composite electrodes. Interestingly, the lithiophilicity of carbon nanofibers varied with the carbonization temperature because of the difference in the surface carbonaceous microstructures (seeFigure 2b) [40]. The carbon/lithium composite also showed an improved electrochemical performance compared with that of bare lithium. The silicon coating layer was used to make the carbon nanofiber mat lithophilic [41]. Likewise, the composite electrode exhibited a low volume change as well as a reduced overpotential during electrochemical reactions.
2b) [247]. The carbon/lithium composite also showed an improved electrochemical performance compared with that of bare lithium. The silicon coating layer was used to make the carbon nanofiber mat lithophilic [248]. Likewise, the composite electrode exhibited a low volume change as well as a reduced overpotential during electrochemical reactions.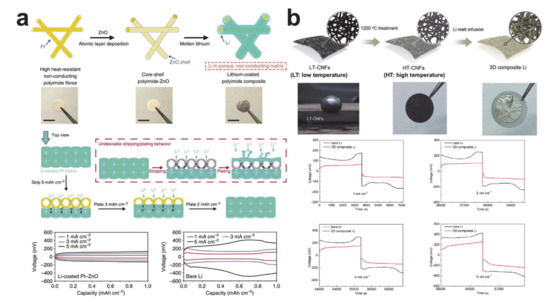
Figure 22.
a) Lithium/zinc oxide coated polyimide composite (reprinted with permission from [38]; copyright 2016 Springer Nature) and (
b) lithium/carbon composite (reprinted with permission from [40]; copyright 2017 Royal Society of Chemistry).
Current lithium-ion batteries are operated only with the lithium initially contained in the cathode material. In other words, there is no additional source of lithium other than the cathode. This means that the lithium metal host does not have to contain lithium if the ideal case is assumed and the reaction is reversible. However, electrochemical performances of real lithium-free anode cells deteriorate owing to the SEI formation and dead lithium formation with non-uniform lithium plating and stripping [42]. Thus, regulating the lithium deposition behavior is crucial to improve the rechargeability of the lithium metal anodes.
Current lithium-ion batteries are operated only with the lithium initially contained in the cathode material. In other words, there is no additional source of lithium other than the cathode. This means that the lithium metal host does not have to contain lithium if the ideal case is assumed and the reaction is reversible. However, electrochemical performances of real lithium-free anode cells deteriorate owing to the SEI formation and dead lithium formation with non-uniform lithium plating and stripping [249]. Thus, regulating the lithium deposition behavior is crucial to improve the rechargeability of the lithium metal anodes. shows various designs of the lithium plating scaffold produced from electrospun nanofibers. The semi-tubular carbon film inFigure 23a was synthesized by templating an electrospun PVP nanofiber mat [43]. The carbon film guided homogenous lithium to be deposited underneath the film in the absence of Li dendrites. As a result, overpotentials of the carbon film-coated Cu|Li asymmetrical cell after the first cycle were much lower than those of the bare Cu|Li cell. Research has been undertaken on more delicate mechanisms to control the lithium plating behavior. As shown in
a was synthesized by templating an electrospun PVP nanofiber mat [250]. The carbon film guided homogenous lithium to be deposited underneath the film in the absence of Li dendrites. As a result, overpotentials of the carbon film-coated Cu|Li asymmetrical cell after the first cycle were much lower than those of the bare Cu|Li cell. Research has been undertaken on more delicate mechanisms to control the lithium plating behavior. As shown inFigure 23b, a gold coating layer was employed on the back side (the side away from the separator) to encourage selective deposition of lithium ions through homogeneous seeded growth using gold nanoparticles as seeds [44]. The gold-coated carbon nanofibers exhibited a lower overpotential and longer cyclability. Likewise, a mixed ion- and electron-conducting network consisting of a superionic conducting material (Li
b, a gold coating layer was employed on the back side (the side away from the separator) to encourage selective deposition of lithium ions through homogeneous seeded growth using gold nanoparticles as seeds [251]. The gold-coated carbon nanofibers exhibited a lower overpotential and longer cyclability. Likewise, a mixed ion- and electron-conducting network consisting of a superionic conducting material (Li6.4
La3
Zr2
Al0.2
O12
(LLZO)) and an excellent electronic conducting material (carbon) was designed for homogeneous plating and rapid stripping of lithium (Figure 23c) [45], and ultrafine silver nanoparticles with a carbon composite nanofiber were synthesized using the Joule heating method to guide preferential lithium plating on the carbon nanofiber (
c) [252], and ultrafine silver nanoparticles with a carbon composite nanofiber were synthesized using the Joule heating method to guide preferential lithium plating on the carbon nanofiber (Figure 23d) [46]. Despite such impressive progress on the plating/stripping anode materials, there are still significant margins of improvement in advancing lithium metal batteries.
d) [253]. Despite such impressive progress on the plating/stripping anode materials, there are still significant margins of improvement in advancing lithium metal batteries.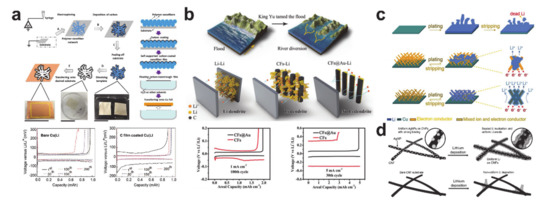

Figure 23.
a) Semi-tubular carbon film (reprinted with permission from [43]; copyright 2017 Elsevier), (
b) gold-coated carbon nanofiber (reprinted with permission from [44]; copyright 2018 John Wiley and Sons), (
c
6.4
3
2
0.2
12 nanoparticle incorporated carbon nanofiber (reprinted with permission from [45]; copyright 2018 John Wiley and Sons), and (
d) silver nanoparticles decorated carbon nanofiber (reprinted with permission from [46]; copyright 2017 John Wiley and Sons).
4. Current Limitations and Prospects
Firstly, the legacy of electrospun nanofiber anode materials must be used to contribute to the improvement of commercial lithium-ion batteries. There should be an appreciation for the progress made in electrospun nanofiber anode material development; novel concepts, optimum structures, and significant performance improvements have been made via developments in the electrospinning process. On the basis of current progress, it is now viable to implement nanofiber-based anode materials into conventional lithium-ion batteries, primarily owing to developments in insertion, conversion, and alloying anode materials that are proven and ready. There are many papers showing results that target and offer improvements to current commercial lithium-ion batteries, such as higher energy densities, higher power densities, and satisfactory rapid charging performances. However, a large number of parameters must meet the specification requirements for a new electrode material to be implemented in commercial batteries; for example, the loading level (areal specific active material weight), electrode porosity and density, electrode volume change, initial coulombic efficiency, long-term cyclability, high rate performance, operating temperature, and so on. It is extremely difficult to satisfy all the specification requirements; therefore, it may be more reasonable to make small improvements by adding electrospun nanofiber anode materials to existing graphite electrode production. Secondly, greater effort needs to be put into developing lithium metal anodes. The lithium metal anode is the ultimate anode system enabling super-high energy density. As previously mentioned, using a porous electrospun mat is structurally beneficial to storing lithium by plating, and the wide variety of material species that can be applied to the product is advantageous. There are many great opportunities to develop lithium metal anodes using the electrospinning process.Thirdly, the acceleration of solid-state battery development can be encouraged through active studies of nanofiber anode materials. Active research is being conducted on solid-state batteries to consider factors such as enhancing the safety and improving the energy density of conventional lithium-ion batteries by utilizing organic electrolytes [47]. However, very few studies are being conducted on the nanofiber-based solid-state battery anodes [48][49][50]. Identifying the optimum electrode materials and structures should be highly encouraged as this may unearth a totally different ionic transport mechanism, and different interfacial interactions between electrolytes and active materials or cell fabrication processes. The electrospinning process and resultant nanofiber can provide a screening platform to optimize the active material and electrode structure for solid-state battery anodes.
Thirdly, the acceleration of solid-state battery development can be encouraged through active studies of nanofiber anode materials. Active research is being conducted on solid-state batteries to consider factors such as enhancing the safety and improving the energy density of conventional lithium-ion batteries by utilizing organic electrolytes [254]. However, very few studies are being conducted on the nanofiber-based solid-state battery anodes [186,255,256]. Identifying the optimum electrode materials and structures should be highly encouraged as this may unearth a totally different ionic transport mechanism, and different interfacial interactions between electrolytes and active materials or cell fabrication processes. The electrospinning process and resultant nanofiber can provide a screening platform to optimize the active material and electrode structure for solid-state battery anodes.5. Concluding Remarks
Electrospun anode materials were designed to improve the desired properties and to reduce the negative properties based on the electrochemical reaction type being studied. Some of the more prominent achievements are already at a high technology-readiness level and are ready to be implemented in commercial lithium-ion battery production; furthermore, forming a graphite composite with the existing electrode can reduce the difficulty barrier to introducing nanofiber-based anode materials. More effort is required with regard to promoting the use of anode materials for lithium metal batteries and solid-state batteries, in order to accelerate next-generation battery development.
