This narrative review focuses on the contributing factors and health implications of night eating during pregnancy, based on evidence from cross-sectional studies and longitudinal cohorts. The modern lifestyle and presence of pregnancy symptoms contribute to night eating during pregnancy, which is likely to coexist and interact with multiple undesirable lifestyle behaviors. Unfavorable nutritional characteristics associated with night eating have the potential to induce aberrant circadian rhythms in pregnant women, resulting in adverse metabolic and pregnancy outcomes.
- chrononutrition
- pregnancy
- meal timing
- night eating
- circadian rhythm
- lifestyle behavior
1. Introduction
The timing of eating has become an important area of research given the increasing recognition of food intake as a zietgeber (time cue) for the circadian system of humans [1]. Eating food at times that contradict body’s natural circadian rhythms, such as eating during the inactive/sleep phase, has been shown to entrain the expression of clock genes in most peripheral tissues, e.g., liver, pancreas, skeletal muscle, adipose tissue, leading to misalignment between internal circadian rhythms [2][3]. Consequently, there is a potential adverse effect on metabolic physiology, increasing the risk of diabetes, obesity, cardiovascular disease and possibly even cancer [4][5][6]. Thus, desynchronization between the light-entrainable central clock in the brain and food-entrainable peripheral clocks in tissues appears to be disadvantageous for metabolism [7]. This refers to the concept of chrononutrition—the study of food’s impacts on metabolism via the circadian clock system [8]. Chrononutrition reflects the basic concept that not only food quantity and quality, but also food timing is critical for the well-being of an individual [9].
Maternal adaption in pregnancy induces changes in circadian rhythms [10] with marked alterations in the expression of circadian clock genes [11][12]. More specifically, changes in maternal peripheral clock gene expressions across pregnancy drive downstream shifts in circadian expression of certain metabolic genes, such as glucoregulatory genes Pck1, G6Pase and Gk to support healthy pregnancy [12]. This indicates that when circadian rhythms during pregnancy are disrupted, there is a potential for putting women at risk of developing metabolic disorders and adverse pregnancy outcomes. There is established evidence that pregnant night-shift workers are at risk of miscarriage, prematurity, low birth weight and hypertensive disorders [13][14]. These findings have relevance to not only pregnant night-shift workers, but may also apply to the general pregnant population who consume high energy intakes in the evening or at night with potential chronodisruption. Night-time is a period when the body is naturally primed for rest in humans.
2. Potential Reasons for Maternal Night Eating
It is unclear whether maternal night eating is a behavior established before conceiving, or exaggerated or exhibited only during pregnancy. This information is important to determine the target groups and time-point of interventions addressing night eating (i.e., preconception or trimester specific during pregnancy). Understanding potential reasons for maternal night eating during pregnancy might provide some clues on this aspect, and useful baseline information in developing intervention strategies.
Lifestyle habits and time pressure have been frequently cited as contributors to unhealthy eating, including night eating [15]. Past studies have associated long working hours and shift work with irregular meal timing. Escoto et al. [16] studied the relationship between the number of working hours per week and time-related beliefs to healthy eating among 2287 American adults. The study reported that individuals with longer working hours (>40 h per week) often pay little attention to nutritional balance and have late dinners [16]. A study in South Korea involving 340 nurses found that those with rotating night-shift schedules were more often engaged in breakfast skipping and late night snacking, as compared to nurses without night-shifts [17]. Thus, it is expected that the same scenario can occur in working women who are pregnant. However, compared to before pregnancy, women may be more likely to practice night eating after becoming pregnant due to the presence of pregnancy symptoms and discomforts [18], which could disrupt their eating patterns [19].
To our knowledge, only one study to date has explored the reasons for night eating among pregnant women. Based on a qualitative survey involving 18 pregnant African American women (27–38 weeks gestation) who regularly ate between 8:00 p.m. and 6:00 a.m., altered sleep schedules, hunger, thirst, nausea, fetal movement and personal choice were the common reasons for night eating [20]. These women reported having difficulty sleeping at night, which caused them to wake up later in the morning and delay their entire meal timing throughout the day [20]. This is supported by a study conducted in 266 Polish women (28–41 weeks gestation), showing that night eating was associated with insomnia during pregnancy, although any causal-effect relationship is unclear [21]. Forty percent of women who developed insomnia during pregnancy reported constantly waking up at night to eat [21]. Since insomnia occurs most frequently during the third trimester of pregnancy [22][23], this may be accompanied with more frequent night eating.
In a qualitative survey by Kroeger et al. [20], a number of women reported constantly feeling hungry during pregnancy. Some of them regularly woke up at a specific time during the night to eat and drink due to hunger and thirst. However, the role of appetite in night waking behavior during pregnancy is uncertain. It has been proposed that such a phenomenon may plausibly be due to impaired fat oxidation in these women [20]. Fatty acids are the main energy source during overnight fasting [24][25]. There is evidence that individuals who engage in night eating have decreased fat oxidation [26][27][28]. Although fat oxidation was found to be downregulated among obese individuals [29] and those who are prone to obesity [30], little is known about whether there is downregulation of fat oxidation during pregnancy, which could contribute to hunger at night.
It has been documented that pregnant women tend to experience nausea in the morning, causing them to eat less during the day but more at night [20]. This was supported by a study in Brazil following pregnant women from 4 to 37 weeks gestation, showing breakfast skipping was more prevalent in night eaters than day eaters throughout pregnancy [31]. Another study involving Norwegian pregnant women at 17–22 weeks gestation reported that nausea was positively associated with an evening meal pattern tendency [32]. In addition to nausea, low appetite in the morning might also cause women to delay their temporal distribution of food intake. In a clinical trial examining circadian influences on appetite among 12 healthy adults, the circadian rhythm in hunger reached a trough at 8 a.m., indicating appetite was clock-controlled and reached at its lowest in the morning [33].
Night-time fetal movement is common during the third trimester of pregnancy; a few studies have demonstrated that fetal movement increased along the day, with peak activity between 9:00 p.m. and 1:00 a.m. [34][35]. Some pregnant women have interpreted fetal movement as a fetal demand for food, prompting them to wake up and eat [20][36].
On top of pregnancy symptoms and physiological reasons, night eating during pregnancy has been reported to be influenced by personal preference and psychosocial factors [20]. For personal preference, it is consistent with the Theory of Planned Behavior, indicating that individuals often hold a belief or attitude about eating whenever they want to rather than adhering to a fixed eating schedule [37]. This applies to food craving, which is common during pregnancy [19], and craving frequency has been associated with increased night snacking [38]. The influence of other members in the household was cited as the primary psychosocial influence on night eating [20]. This suggests that the likelihood of engaging in night eating is related to an individual’s beliefs and household environment.
3. Night Eating and Maternal Health Outcomes
There is evidence of impaired glucose metabolism in response to night eating among pregnant women [39][40], which is likely attributable to relatively low insulin sensitivity during this time [41]. However, a weight-dependent effect was observed. A study by Chandler-Laney et al. [39] showed that independent of daytime energy intake, increased night-time energy intake was associated with reduced dynamic β-cell response in the obese pregnant women (pre-pregnancy BMI ≥ 30 kg/m2), but not in those who were of a normal weight (pre-pregnancy BMI < 25 kg/m2). In contrast, a later study by Loy et al. [40] reported that independent of TDEI, pregnant night eaters who were lean (pre-pregnancy BMI < 23 kg/m2) had an increased fasting plasma glucose, but this association was not found in those who were overweight/obese (pre-pregnancy BMI ≥ 23 kg/m2). The authors proposed that this discrepancy may be due to the marked suppression of insulin sensitivity in the morning among overweight/obese individuals, leading to failure detection for a further reduction in insulin sensitivity [40]. In the aforementioned study [39], when further examination of night-time carbohydrate intake was performed, independent of TDEI, a high carbohydrate intake at night instead of during the day was associated with reduced glucose tolerance and lowered insulin secretion in obese pregnant women. This finding is supported by an earlier study which revealed that night snacking of carbohydrate-rich foods was more often observed in women developing gestational diabetes mellitus (GDM) than those with normal glucose tolerance [42]. Observations in pregnant women are in agreement with evidence in adults, indicating that food timing and the amount of dietary carbohydrate could affect glucose metabolism [43]. Indeed, dietary carbohydrate composition (e.g., highly vs. poorly digestible carbohydrate) has been shown to heavily influence the clock in regulating glucose homeostasis [44]. Taken together, maternal night-time energy intake and specifically, the amount and type of night-time carbohydrate intake have the potential to affect glucose tolerance and β-cell function during pregnancy, and the risk of GDM development.
A prospective cohort study reported that compared to pregnant women with lower night-time energy intake, those with higher night-time energy intake had an adverse pattern of gestational weight gain (GWG) in the third trimester, independent of pre-pregnancy BMI [31]. Of note, there were no differences in TDEI and macronutrient intake in the third trimester between these groups of pregnant women [31]. Another prospective cohort study further demonstrated that night eating during pregnancy was associated with greater weight retention of at least 5 kg at 18 months postpartum, independent of TDEI and early pregnancy BMI [45]. GDM, GWG and breastfeeding practice after delivery did not alter this association [45]. Based on this observation, the authors suggested the possibility of persistent night eating behavior beyond pregnancy, with implications for long-term obesity risk [45]. These findings are consistent with a meta-analysis of observational studies in adults, reporting an association between greater night-time energy intake and higher BMI [46]. Given that night time is a period with delayed gastric emptying [47], decreased thermic effect of food and reduced resting metabolic rate [48], consuming a large amount of energy during this period may be detrimental to metabolic processes during pregnancy by dysregulating circadian rhythms, disrupting hormone secretion and altering gut microbiome [2][49].
Aside from metabolic implications, maternal night eating has been associated with the duration of gestation. Pregnant night eaters in the second trimester were reported to exhibit a shorter gestation and a greater likelihood of delivering preterm (<37 weeks gestation), independent of TDEI, sociodemographic and lifestyle factors [50]. This association was not altered by bedtime or glycemic measures during pregnancy [50]. The study provided new evidence on the role of night eating in preterm delivery. Indeed, this finding is supported by the Nurses’ Health Study, which demonstrated an increased risk of preterm birth in nurses working night-shifts, most likely explained by circadian disruption [51]. It has been reported that consuming foods at night may suppress melatonin [52] due to circadian misalignment [3], leading to dysregulation of oxytocin, uterine contractility and birth timing [53]. Although the shift or delay in meal timing during the rest phase is less severe in night eaters compared to shift workers, pregnant women with night eating may remain exposed to a milder form of chronodisruption that contributes to preterm birth. In contrast, evening meal pattern during mid-pregnancy as assessed in the MoBa study was not associated with preterm birth; however, the study did not evaluate night-time energy intake [32].
4. Concluding Perspectives
To better evaluate and understand maternal night eating, the use of multiple-day food diaries or 24 h recalls across varying trimesters of pregnancy is an ideal approach. In addition to being more reliable, it enables the evaluation of night eating behavior changes throughout pregnancy. Importantly, using a food diary or recall allows flexibility of using different time frames and different energy intake cut-offs to define night eating and night eaters according to the local context, e.g., nightfall period and dinner time, as geographical and cultural differences are present in the temporal distribution of eating. Although this method may hinder data harmonisation for the comparison of findings, it allows us to understand how a certain proportion of TDEI and diet composition during a specific period of the night within a population could impact on health. This facilitates the design and development of dietary interventions based on meal timing which is socially and culturally appropriate.
The current review focused on providing an overview of the potential contributing factors and health effects of night eating during pregnancy based on evidence from observational studies (Figure 1). Targeting nutrition as a strategy to optimize pregnancy outcomes is an important research agenda for scientists and dietitians/nutritionists. Timing of energy intake and diet composition appear as potential novel approaches to address metabolic complications during pregnancy and adverse birth outcomes. Based on the limited numbers of studies discussed, high night-time energy intake and its nutritional profile may play a role in contributing to impaired energy and glucose metabolism, and reproductive hormone disruption during pregnancy. In other words, it seems that managing the amount of energy intake and food choice during the night may offer part of the solution to reducing adverse pregnancy outcomes. Intervention trials are warranted to provide more definitive information in this field. Food types, attitude, behavioral components (e.g., lifestyle and chronotype) and pregnancy symptoms relating to night eating are important aspects to be considered in the design of intervention that aims at addressing night eating. This will help to facilitate adherence and to better predict the effectiveness of a given intervention by accounting for these factors in the analysis. If the results are promising, management strategies for night eating should be addressed in nutrition guidelines and counselling for pregnant women.
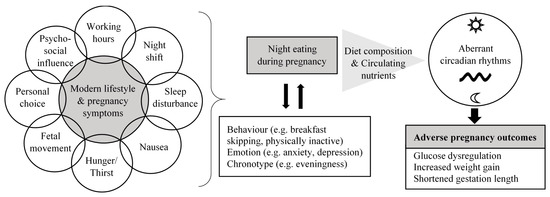
To have better understanding of how night eating relates to health, both evening meals and nocturnal snacks should be analyzed separately due to the potential variation in metabolic implications. A snack, defined as a feeding event not motivated by physiological hunger but elicited by an external non-physiological stimulus, has been proposed to exhibit different energy substrate utilization from taking a meal, which is triggered by hunger [54]. Increasing evidence suggests that negative metabolic implications may not occur if the bedtime snack is low in calories and rich in certain nutrients such as protein [55]. In addition, in studying food timing, components including meal (ir)regularity (meal skipping/delaying), eating frequency (number of meals and snacks) and fasting interval (duration of daytime or night-time fasting, spacing of eating) should be taken into account as they are inter-connected. In a recent large-scale clinical trial using machine-learning to predict human postprandial responses to food intake, both meal timing and periods of fasting have been proposed to offer a large potential for improving the prediction of postprandial responses linked with cardiometabolic disease [56][57].
Nevertheless, most aforementioned components have been scarcely explored in pregnant women. A few epidemiological studies have reported that pregnant meal skippers may be at risk of preterm delivery [32][58][59][60]. In particular, breakfast skipping in early pregnancy was associated with offspring obesity development through the first 12 years of life [61][62]. However, it is not known whether the observed outcome was contributed by breakfast skipping or because of night eating, given the positive association between these two variables [31]. This suggests a need for future studies to take into account night-time energy intake to determine the relative benefit of breakfast eating compared to night eating. In addition, compared to dinner skipping, whether breakfast skipping imposes a more detrimental health effect remains an unanswered question. This is built on the basis of hypothesized differential phase effects (day and night) of fasting period according to natural circadian rhythms on metabolic regulation.
As most existing studies have focused on trimester-specific dietary assessment, prospective cohorts are needed that assess maternal diet using multiple food diaries or recalls across trimesters, including during the preconception phase. This will allow the investigation of meal pattern changes and specific windows when circadian eating may have started to impose a detrimental effect on pregnancy outcomes. Advanced technologies, such as using mobile phone apps to record diet and food images with time-stamps, and the wearing of continuous recording devices to measure 24-h blood glucose and activity, will provide valuable information to aid understanding of how temporal eating patterns influence physiological processes and health. Importantly, investigations on mechanistic pathways underlying night eating and maternal-fetal health outcomes, immediately and long-term, are required. In pregnant rodents, disrupted food timing (eating during the day for nocturnal animals) has been shown to cause aberrant circadian rhythmicity in both the mother and fetus [63], and could potentially modify microbiota profiles in ways that lead to metabolic disorders [49], but the translation of these mechanisms to pregnant women has yet to be performed.
References
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a circadian time cue—Evidence from human studies. Nat. Rev. Endocrinol. 2020, 16, 213–223, doi:10.1038/s41574-020-0318-z.
- Johnston, J.D.; Ordovás, J.M.; Scheer, F.A.J.L.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans123. Adv. Nutr. 2016, 7, 399–406, doi:10.3945/an.115.010777.
- Guerrero-Vargas, N.N.; Espitia-Bautista, E.; Buijs, R.M.; Escobar, C. Shift-work: Is time of eating determining metabolic health? Evidence from animal models. Proc. Nutr. Soc. 2018, 77, 199–215, doi:10.1017/s0029665117004128.
- Moran-Ramos, S.; Baez-Ruiz, A.; Buijs, R.M.; Escobar, C. When to eat? The influence of circadian rhythms on metabolic health: are animal studies providing the evidence? Nutr. Res. Rev. 2016, 29, 180–193, doi:10.1017/s095442241600010x.
- McHill, A.W.; Phillips, A.J.; A Czeisler, C.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219, doi:10.3945/ajcn.117.161588.
- Srour, B.; Plancoulaine, S.; Andreeva, V.A.; Fassier, P.; Julia, C.; Galan, P.; Hercberg, S.; Deschasaux, M.; Latino-Martel, P.; Touvier, M. Circadian nutritional behaviours and cancer risk: New insights from the NutriNet‐santé prospective cohort study: Disclaimers. Int. J. Cancer 2018, 143, 2369–2379, doi:10.1002/ijc.31584.
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152, doi:10.1016/j.molmet.2015.12.006.
- Pot, G.K.; Almoosawi, S.; Stephen, A.M. Meal irregularity and cardiometabolic consequences: results from observational and intervention studies. Proc. Nutr. Soc. 2016, 75, 475–486, doi:10.1017/s0029665116000239.
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92, doi:10.1016/j.cell.2015.03.015.
- Martin-Fairey, C.A.; Zhao, P.; Wan, L.; Roenneberg, T.; Fay, J.; Ma, X.; McCarthy, R.; Jungheim, E.S.; England, S.K.; Herzog, E.D. Pregnancy Induces an Earlier Chronotype in Both Mice and Women. J. Biol. Rhythm. 2019, 34, 323–331, doi:10.1177/0748730419844650.
- Wharfe, M.D.; Mark, P.J.; Wyrwoll, C.S.; Smith, J.T.; Yap, C.; Clarke, M.W.; Waddell, B.J. Pregnancy-induced adaptations of the central circadian clock and maternal glucocorticoids. J. Endocrinol. 2015, 228, 135–147, doi:10.1530/joe-15-0405.
- Wharfe, M.D.; Wyrwoll, C.S.; Waddell, B.J.; Mark, P.J. Pregnancy-induced changes in the circadian expression of hepatic clock genes: implications for maternal glucose homeostasis. Am. J. Physiol. Metab. 2016, 311, E575–E586, doi:10.1152/ajpendo.00060.2016.
- Gamble, K.L.; Resuehr, D.; Johnson, C.H. Shift Work and Circadian Dysregulation of Reproduction. Front. Endocrinol. 2013, 4, doi:10.3389/fendo.2013.00092.
- Hammer, P.; Flachs, E.; Specht, I.O.; Pinborg, A.; Petersen, S.; Larsen, A.; Hougaard, K.; Hansen, J.; Hansen, Å.; Kolstad, H.; et al. Night work and hypertensive disorders of pregnancy: a national register-based cohort study. Scand. J. Work. Environ. Health 2018, 44, 403–413, doi:10.5271/sjweh.3728.
- Mc Morrow, L.; Ludbrook, A.; MacDiarmid, J.; Olajide, D. Perceived barriers towards healthy eating and their association with fruit and vegetable consumption. J. Public Heal. 2016, 39, 330–338.
- Escoto, K.H.; Laska, M.N.; Larson, N.; Neumark-Sztainer, D.; Hannan, P.J. Work hours and perceived time barriers to healthful eating among young adults. Am. J. Heal. Behav. 2012, 36, 786–796.
- Han, K.; Choi-Kwon, S.; Kim, K.S. Poor dietary behaviors among hospital nurses in Seoul, South Korea. Appl. Nurs. Res. 2016, 30, 38–44.
- Sayle, A.E.; Wilcox, A.J.; Weinberg, C.R.; Baird, D.D. A prospective study of the onset of symptoms of pregnancy. J. Clin. Epidemiol. 2002, 55, 676–680.
- Forbes, L.; Graham, J.; Berglund, C.; Bell, R. Dietary Change during Pregnancy and Women’s Reasons for Change. Nutrients 2018, 10, 1032.
- Kroeger, E.; Carson, T.L.; Baskin, M.L.; Langaigne, A.; Schneider, C.; Bertrand, B.; Herbey, I.I.; Harper, L.M.; Biggio, J.; Chandler-Laney, P.C. Reasons for Late-Night Eating and Willingness to Change: A Qualitative Study in Pregnant Black Women. J. Nutr. Educ. Behav. 2019, 51, 598–607, doi:10.1016/j.jneb.2018.11.003.
- Wołyńczyk-Gmaj, D.; Różańska-Walędziak, A.; Ziemka, S.; Ufnal, M.; Brzezicka, A.; Gmaj, B.; Januszko, P.; Fudalej, S.; Czajkowski, K.; Wojnar, M. Insomnia in Pregnancy is Associated with Depressive Symptoms and Eating at Night. J. Clin. Sleep Med. 2017, 13, 1171–1176.
- Kızılırmak, A.; Taşhan, S.T.; Kartal, B. Insomnia in Pregnancy and Factors Related to Insomnia. Sci. World J. 2012, 2012, 1–8.
- Fernández-Alonso, A.M.; Trabalón-Pastor, M.; Chedraui, P.; Pérez-López, F.R. Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch. Gynecol. Obstet. 2012, 286, 55–61.
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Metab. 2008, 295, E1009–E1017.
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450.
- Gluck, M.E.; Venti, C.A.; Salbe, A.D.; Votruba, S.B.; Krakoff, J. Higher 24-h respiratory quotient and higher spontaneous physical activity in nighttime eaters. Obesity 2010, 19, 319–323.
- Hibi, M.; Masumoto, A.; Naito, Y.; Kiuchi, K.; Yoshimoto, Y.; Matsumoto, M.; Katashima, M.; Oka, J.; Ikemoto, S. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R94–R101.
- Kelly, K.P.; McGuinness, O.P.; Buchowski, M.; Hughey, J.J.; Chen, H.; Powers, J.; Page, T.; Johnson, C.H. Eating breakfast and avoiding late-evening snacking sustains lipid oxidation. PLoS Biol. 2020, 18, e3000622.
- Rynders, C.A.; Bergouignan, A.; Kealey, E.; Bessesen, D.H. Ability to adjust nocturnal fat oxidation in response to overfeeding predicts 5-year weight gain in adults. Obesity 2017, 25, 873–880.
- Schmidt, S.L.; Kealey, E.H.; Horton, T.J.; VonKaenel, S.; Bessesen, D.H. The effects of short-term overfeeding on energy expenditure and nutrient oxidation in obesity-prone and obesity-resistant individuals. Int. J. Obes. 2012, 37, 1192–1197.
- Gontijo, C.; Balieiro, L.C.T.; Teixeira, G.P.; Fahmy, W.M.; Crispim, C.A.; Maia, Y.; Araújo, G.C.; Tibiletti, B.L.C.; Pereira, T.G.; Makin, F.W.; et al. Higher energy intake at night effects daily energy distribution and contributes to excessive weight gain during pregnancy. Nutrition 2020, 74, 110756
- Englund-Ögge, L.; Birgisdóttir, B.E.; Sengpiel, V.; Brantsæter, A.L.; Haugen, M.; Myhre, R.; Meltzer, H.M.; Jacobsson, B. Meal frequency patterns and glycemic properties of maternal diet in relation to preterm delivery: Results from a large prospective cohort study. PLoS ONE 2017, 12, e0172896.
- Scheer, F.A.J.L.; Morris, C.J.; Shea, S. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity 2013, 21, 421–423.
- Patrick, J.; Campbell, M.K.; Carmichael, L.; Natale, R.; Richardson, B. Patterns of gross fetal body movements over 24-hour observation intervals during the last 10weeks of pregnancy. Am. J. Obstet. Gynecol. 1982, 142, 363–371.
- Stone, P.; Burgess, W.; McIntyre, J.P.R.; Gunn, A.J.; Lear, C.A.; Bennet, L.; Mitchell, E.A.; Thompson, J.M.D. An investigation of fetal behavioural states during maternal sleep in healthy late gestation pregnancy: An observational study. J. Physiol. 2017, 595, 7441–7450.
- Bradford, B.; Maude, R. Fetal response to maternal hunger and satiation—Novel finding from a qualitative descriptive study of maternal perception of fetal movements. BMC Pregnancy Childbirth 2014, 14, 1–9.
- McDermott, M.S.; Oliver, M.; Svenson, A.; Simnadis, T.; Beck, E.J.; Coltman, T.; Iverson, D.; Caputi, P.; Sharma, R. The theory of planned behaviour and discrete food choices: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–11.
- Orloff, N.C.; Flammer, A.; Hartnett, J.; Liquorman, S.; Samelson, R.; Hormes, J.M. Food cravings in pregnancy: Preliminary evidence for a role in excess gestational weight gain. Appetite 2016, 105, 259–265.
- Chandler-Laney, P.; Schneider, C.; Gower, B.A.; Granger, W.M.; Mancuso, M.S.; Biggio, J. Association of late-night carbohydrate intake with glucose tolerance among pregnant African American women. Matern. Child Nutr. 2015, 12, 688–698.
- Loy, S.L.; Cheng, T.S.; Colega, M.T.; Cheung, Y.B.; Godfrey, K.M.; Gluckman, P.D.; Kwek, K.; Saw, S.M.; Chong, Y.-S.; Padmapriya, N.; et al. Predominantly night-time feeding and maternal glycaemic levels during pregnancy. Br. J. Nutr. 2016, 115, 1563–1570.
- Saad, A.; Man, C.D.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal Pattern to Insulin Secretion and Insulin Action in Healthy Individuals. Diabetes 2012, 61, 2691–2700.
- Park, H.-J.; Lee, J.; Kim, J.-M.; Lee, H.A.; Kim, S.-H.; Kim, Y. A Study of Snack Consumption, Night-Eating Habits, and Nutrient Intake in Gestational Diabetes Mellitus. Clin. Nutr. Res. 2013, 2, 42–51.
- Gallant, A.; Lundgren, J.D.; Drapeau, V. Nutritional Aspects of Late Eating and Night Eating. Curr. Obes. Rep. 2013, 3, 101–107.
- Ribas-Latre, A.; Eckel-Mahan, K. Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health. Mol. Metab. 2016, 5, 133–152.
- Loy, S.L.; Cheung, Y.B.; Colega, M.T.; Chia, A.-R.; Han, C.Y.; Godfrey, K.M.; Chong, Y.-S.; Shek, L.P.; Tan, K.H.; Lek, N.; et al. Associations of Circadian Eating Pattern and Diet Quality with Substantial Postpartum Weight Retention. Nutrients 2019, 11, 2686.
- Fong, M.; Caterson, I.D.; Madigan, C.D. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br. J. Nutr. 2017, 118, 616–628.
- Goo, R.; Moore, J.; Greenberg, E.; Alazraki, N. Circadian variation in gastric emptying of meals in humans. Gastroenterology 1987, 93, 515–518.
- Shaw, E.; Leung, G.K.W.; Jong, J.; Coates, A.M.; Blair, M.; Huggins, C.E.; Huggins, C.E.; Dorrian, J.; Banks, S.; Coates, A.M.; et al. The Impact of Time of Day on Energy Expenditure: Implications for Long-Term Energy Balance. Nutrients 2019, 11, 2383.
- Paoli, A.; Tinsley, G.M.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719.
- Loy, S.L.; Cheung, Y.B.; Cai, S.; Colega, M.T.; Godfrey, K.M.; Chong, Y.-S.; Shek, L.P.-C.; Tan, K.H.; Chong, M.F.-F.; Yap, F.; et al. Maternal night-time eating and sleep duration in relation to length of gestation and preterm birth. Clin. Nutr. 2020, 39, 1935–1942.
- Lawson, C.C.; Whelan, E.A.; Hibert, E.N.; Grajewski, B.; Spiegelman, D.; Rich-Edwards, J.W. Occupational factors and risk of preterm birth in nurses. Am. J. Obstet. Gynecol. 2008, 200, 51.e1–51.e8.
- Valenzuela, F.; Vera, J.; Venegas, C.; Pino, F.; Lagunas, C. Circadian System and Melatonin Hormone: Risk Factors for Complications during Pregnancy. Obstet. Gynecol. Int. 2015, 2015, 1–10.
- Reschke, L.; McCarthy, R.; Herzog, E.D.; Fay, J.C.; Jungheim, E.S.; England, S.K. Chronodisruption: An untimely cause of preterm birth? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 60–67.
- Bellisle, F. Meals and snacking, diet quality and energy balance. Physiol. Behav. 2014, 134, 38–43.
- Kinsey, A.W.; Ormsbee, M.J. The Health Impact of Nighttime Eating: Old and New Perspectives. Nutrients 2015, 7, 2648–2662.
- Brand-Miller, J.C.; Buyken, A. Mapping postprandial responses sets the scene for targeted dietary advice. Nat. Med. 2020, 26, 828–830.
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973.
- Siega-Riz, A.M.; Herrmann, T.S.; Savitz, D.A.; Thorp, J.M. Frequency of Eating During Pregnancy and Its Effect on Preterm Delivery. Am. J. Epidemiol. 2001, 153, 647–652.
- Hennessy, M.D.; Volpe, S.L.; Sammel, M.D.; Gennaro, S. Skipping Meals and Less Walking among African Americans Diagnosed with Preterm Labor. J. Nurs. Sch. 2010, 42, 147–155.
- Hernández-Díaz, S.; Boeke, C.E.; Romans, A.T.; Young, B.; Margulis, A.V.; McElrath, T.F.; Ecker, J.L.; Bateman, B.T. Triggers of spontaneous preterm delivery—Why today? Paediatr. Périnat. Epidemiol. 2014, 28, 79–87.
- Mizutani, T.; Suzuki, K.; Kondo, N.; Yamagata, Z. Association of Maternal Lifestyles Including Smoking During Pregnancy with Childhood Obesity. Obesity 2007, 15, 3133–3139.
- Haga, C.; Kondo, N.; Suzuki, K.; Sato, M.; Ando, D.; Yokomichi, H.; Tanaka, T.; Yamagata, Z. Developmental Trajectories of Body Mass Index Among Japanese Children and Impact of Maternal Factors during Pregnancy. PLoS ONE 2012, 7, e51896.
- Varcoe, T.J.; Gatford, K.L.; Kennaway, D.J. Maternal circadian rhythms and the programming of adult health and disease. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R231–R241.
