Cancer is a chronic disease that is responsible for the high death rate, globally. The administration of anticancer drugs is one crucial approach that is employed for the treatment of cancer, although its therapeutic status is not presently satisfactory. The anticancer drugs are limited pharmacologically, resulting from the serious side effects, which could be life-threatening. Polymer drug conjugates, nano-based drug delivery systems can be utilized to protect normal body tissues from the adverse side effects of anticancer drugs and also to overcome drug resistance. They transport therapeutic agents to the target cell/tissue.
- breast cancer
- lung cancer
- chemotherapy
- polymer-based carriers
- polymer-drug conjugates
1. Introduction
2. Classification of Anticancer Chemotherapeutics Based on Their Mechanism of Actions
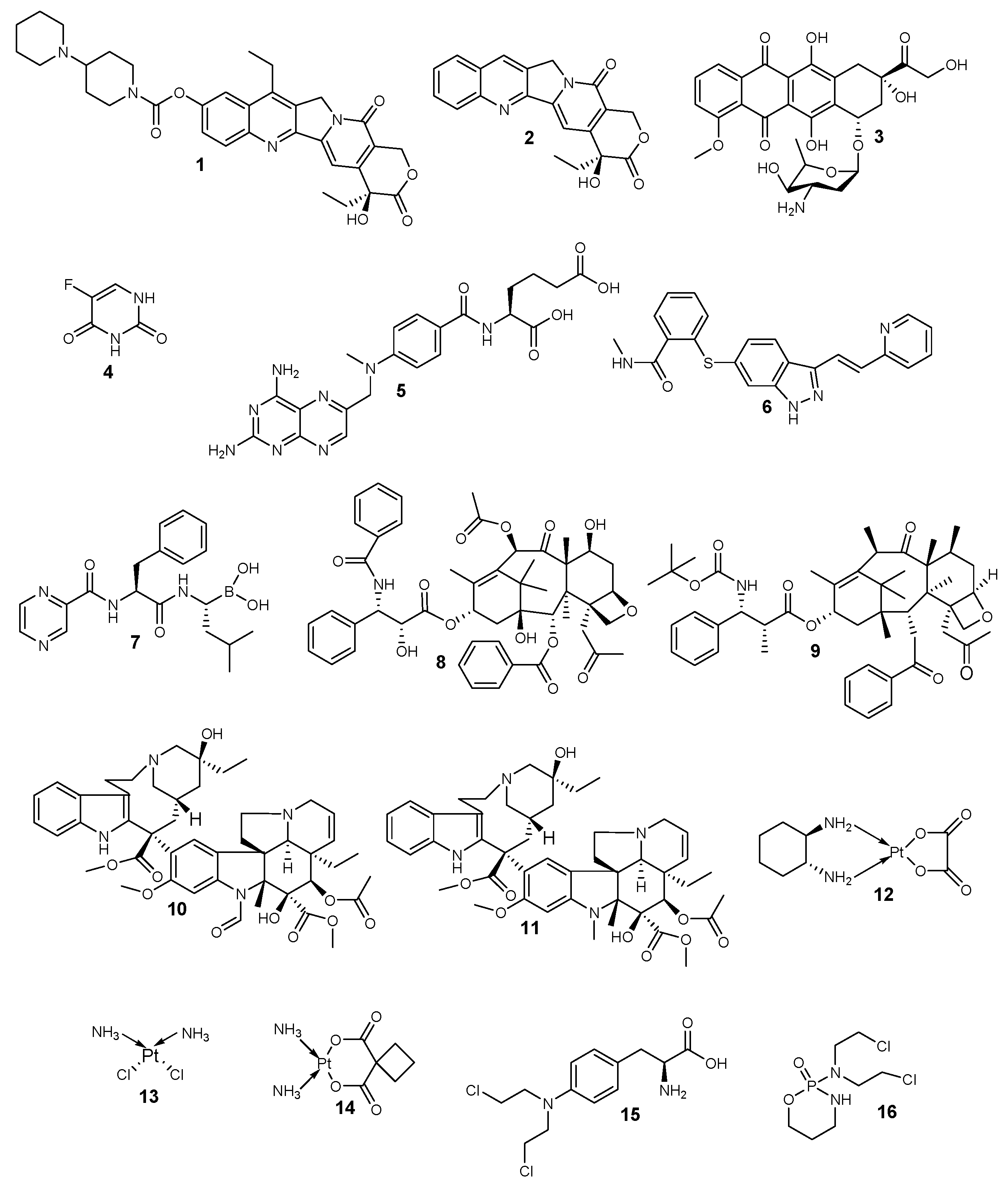
Classes of Anticancer Drugs | Mode of Action | General Mechanisms of Resistance | Examples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Topoisomerase inhibitors | They hinder the binding of the DNA substrate. They also form a cleavage complex, which prevents enzyme turnover and the build-up of high levels of the cytotoxic cleavage complex within the cell. | The altered proliferation and drug targets, reduced sensitivity to apoptosis and cell death, increased ability to repair DNA damage, expression of drug efflux pumps, and detoxification mechanisms. | 1–3 | ||||||||
Antimetabolites | They hinder the biosynthesis of nucleic acids. | 4–7 | |||||||||
Anti-tubulin agents | They disrupt mitotic spindles and terminate mitosis. | 8–11 | |||||||||
Alkylating agents | They bind covalently with the DNA and crosslink them, thereby disrupting the DNA. | 12–16 |
Limitations of Anticancer Drugs and Multi-Drug Resistance
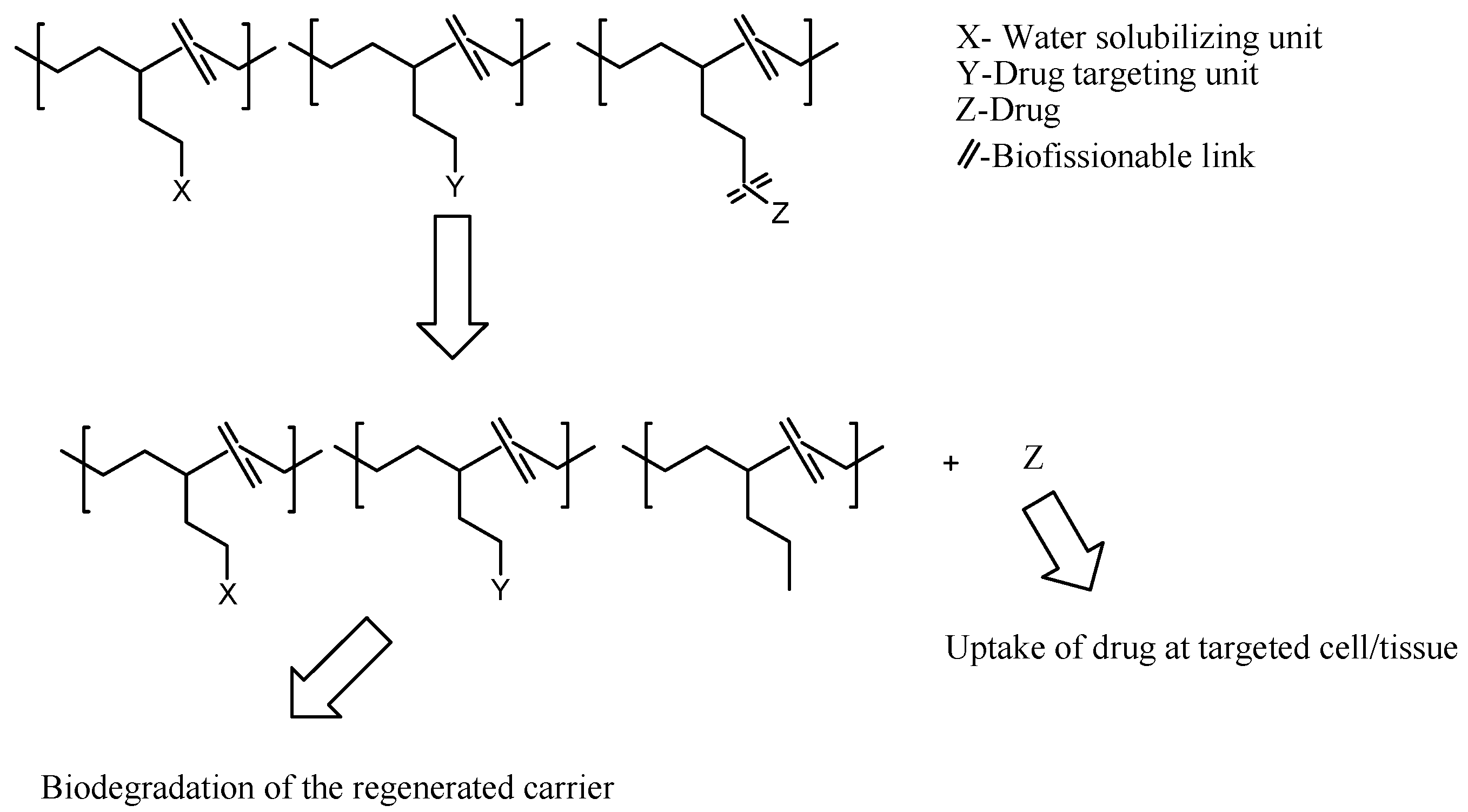
3. Polymer-Drug Conjugates
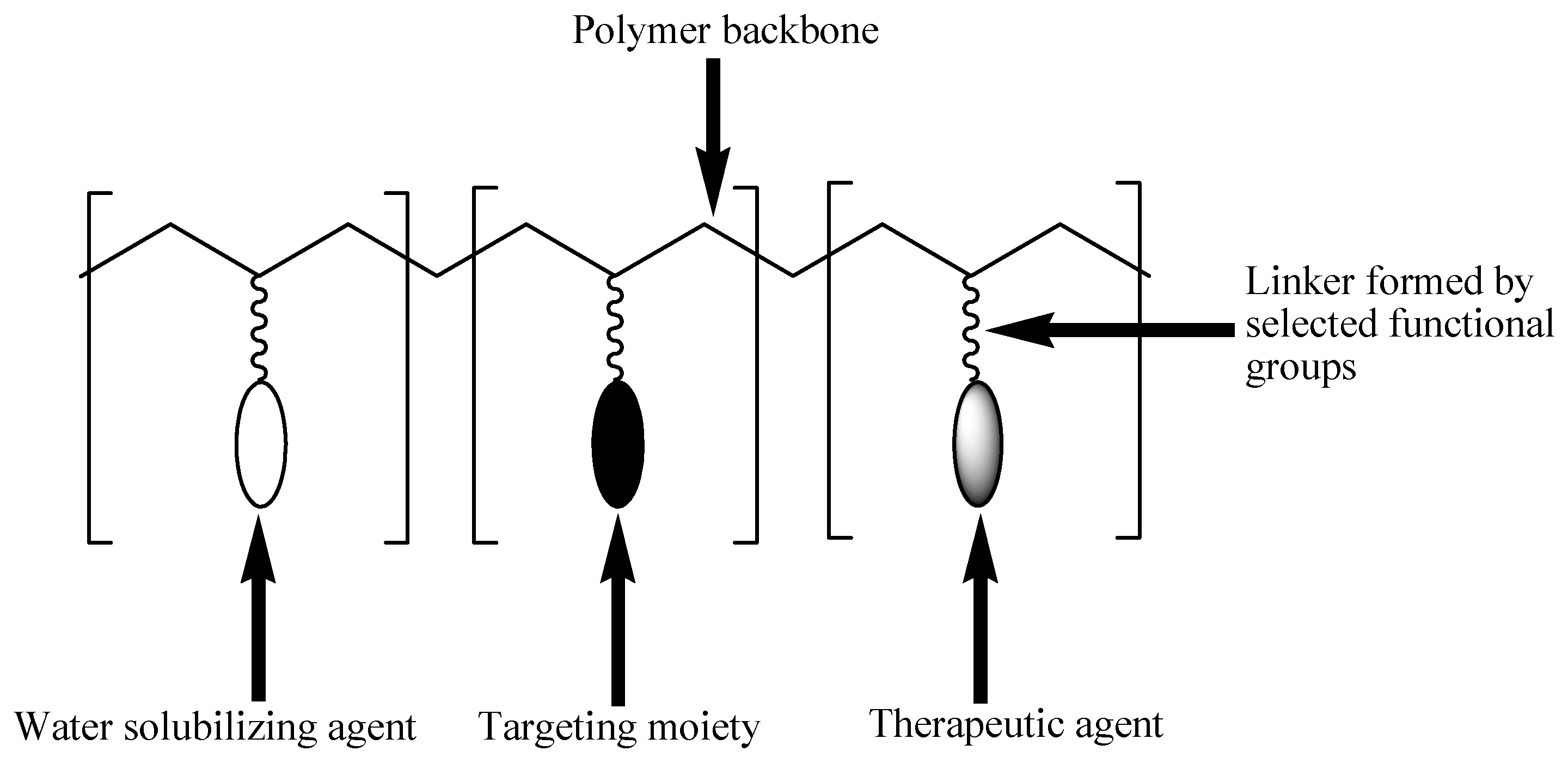
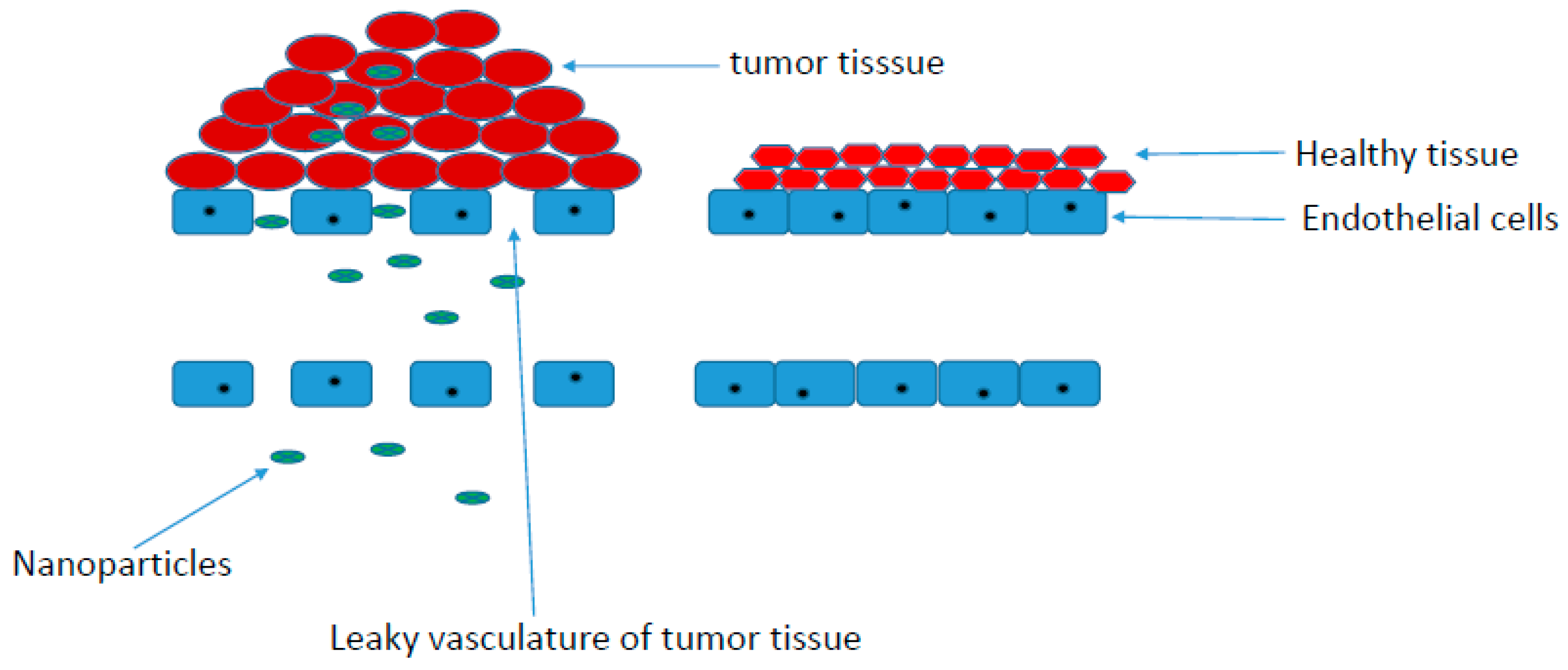
Physicochemical Properties of Polymer-Drug Conjugates for Enhanced Tumor Uptake
4. Polymer-Anticancer Drug Conjugates Effective against Breast Cancer (In Vivo and In Vitro)
Polymer-Drug Conjugates | Carrier/ | Monomers Used | Drugs | Biological Outcomes | Molecular Design | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Polymer-Drug Conjugates | Carrier/Monomers Used | Drugs | Biological Outcomes | Molecular Design | Reference | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
N-(2-hydroxypropyl methyl) acrylamide copolymer-gadolinium-paclitaxel-Cyanine5.5 | N-(2-hydroxypropyl methyl) acrylamide | ||||||||||||||||||||||||||
Hyaluronic acid-dihydroartemisinin (HA-DHA) | Hyaluronic acid | Paclitaxel | Dihydroartemisinin | Mode of administration in vivo: Intravenous. | Prolonged residence time, high accumulation of the conjugate at the tumor site. Inhibition of proliferation and induced apoptosis of the 4T1 murine breast cancer cells. | The conjugates displayed high apoptosis when compared to the free drug | The amphiphilic block polymer was prepared via a two-step Reversible Addition-Fragmentation chain Transfer polymerization and self-assembled into a nanoparticle. Enzyme-sensitive tetrapeptide linker was used as a spacer in the conjugate to promote the degradation of high molecular weight conjugates into low molecular weights with the release of the drug in the cancer microenvironment. | The hydroxyl group of the drug was covalently linked to the carboxylic group of hyaluronic acid. | [111] | ||||||||||||||||||
[ | ] | Hyaluronic acid-Doxorubicin-Gemcitabine | Hyaluronic acid | Doxorubicin and gemcitabine | Mode of administration in vivo: Intravenous and subcutaneous. | The conjugates loaded with both drugs were active in inhibiting the formation of an orthotopic, aggressive 4T1 tumor model in vivo when compared to individual drugs and the polymer-conjugates loaded with a single drug. | The amine on gemcitabine was conjugated to the carboxylic acid on amino acids to form a prodrug. The prodrug was conjugated to hyaluronic acid via carbodiimide chemistry. Doxorubicin was conjugated to hyaluronic acid via carbodiimide chemistry. | ||||||||||||||||||||
Hyaluronic acid-Paclitaxel (HA-PLX) | Hyaluronic acid | [ | Paclitaxel | Significant cytotoxicity and apoptosis-inducing effect resulting from increased cellular uptake of the drug via HA-receptor mediated endocytosis. | Paclitaxel was conjugated to the C-6 position of N-acetyl-D-glucosamine of the hyaluronic acid using hexanediamine as a linker. | 112] | |||||||||||||||||||||
[ | ] | PEG-folic acid-trastuzumab | |||||||||||||||||||||||||
MPEG-b-norbornene functional PLA-b-P(α-BrCL) | Polyethylene glycol | Polylactic acid, Polyethylene glycolFolic acid and trastuzumab | The in vitro cellular uptake of the prodrugs conjugated with both drugs was high when compared to the non-targeted polymeric prodrugs. The conjugate displayed apoptosis of 80% with enhanced tumor regression in vivo. | The copolymer was prepared | by ring-opening polymerization of PEG and lactide followed by isomerization polymerization of the triblock copolymer and 2-hydroxyethyl disulfide using dibutyltin dilaurate as a catalyst. | Doxorubicin and paclitaxel | The incorporation of both drugs into the carrier resulted in a synergistic effect in inhibiting the proliferation of A549 cancer cells. | [113] | |||||||||||||||||||
Both drugs were covalently incorporated into the polymer backbone | [ | 133] |
N-(2-hydroxypropyl) methacrylamide -Doxorubicin | N-(2-hydroxypropyl) methacrylamide | |||||||||||||||||||||||
Polylactide-Paclitaxel (PLA-PTX) | Allyl-functionalized polylactide | Doxorubicin | Paclitaxel | Mode of administration in vivo: Intravenous. | The conjugate exhibited reduced glycolysis, increased apoptosis, and reduced degree of phospholipids when compared to the free doxorubicin. The in vivo studies on the 4T1 breast tumor mouse model using the conjugate revealed a high reduction in the growth of tumors when compared to free DOX-treated mice. | DOX was incorporated into the carriers, and enzyme-sensitive tetrapeptide linker was used as a spacer in the conjugate to promote the degradation of high molecular weight conjugates into low molecular weights with the release of the drug in the cancer microenvironment. | Enhanced cytotoxic effect in vitro. | [114] | |||||||||||||||||||
A polymer-drug conjugate was also obtained by thiol-ene reaction of both thiol-functionalized SB and PTX with allyl-functionalized PLA. | [ | 134] |
Poly-l-glutamic acid-Doxorubicin-Aminoglutethimide | Poly-l-glutamic acid | |||||||||||||||||||||||
Polyethylene glycol-Paclitaxel (PEG-PTX) | Polyethylene glycol | Doxorubicin and aminoglutethimide | Mode of administration in vivo: Intravenous. | The conjugates displayed enhanced tumor cell death and inhibited tumor-related activities. However, the conjugates containing [ N-ε-maleimidocaproic acid hydrazide] moiety displayed a higher survival rate and pro-apoptotic activity, lower anti-apoptotic signals, and inhibition of metastasis. | The conjugates loaded with Dox and aminoglutethimide were prepared with pH-sensitive linkers—hydrazine moiety or complex EMCH [N-ε-maleimidocaproic acid hydrazide] moiety—for the release of Dox in the tumor microenvironment. | [115] | |||||||||||||||||||||
Paclitaxel | The conjugates exhibited sustained drug release with anti-tumor activity, which was less than the free drugs. | The conjugates were prepared with either an azide linker or a succinic linker. The linear PEGs were modified with PTX at the hydroxyl. PTX was incorporated into the PEG molecule via an ester bond at the C-2′ position on the PTX side chain. |
[135] |
Polyethylene glycol -Doxorubicin (PEG-DOX) | |||||||||||||||||||||||
N-(2-hydroxypropyl)methacrylamide-Doxorubicin | Polyethylene glycol | Doxorubicin | Mode of administration in vivo: Intraductal. | Increased molecular weight and decreased branching prolonged the retention of the drug in the mammary gland after administration. | Dox was conjugated to PEG polymers with varied molecular weights (5, 10, 20, and 40 kDa) and architectures of linear, four-arm, and eight-arm. |
N-(2-hydroxypropyl)methacrylamide | Doxorubicin | High cytotoxic activity against the lung cancer cells, which were 10-fold higher cytotoxic against B16-F10, 3LL, and HT29 cells when compared to peptide-doxorubicin. | Doxorubicin was incorporated into N-(2-hydroxypropyl)methacrylamide. | [116] | |||||||||||||||||
[ | ] | Poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) (PLG-g-Mpeg-PTT | |||||||||||||||||||||||||
Poly-l | Poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) | -lysine-lipoic acid-Doxorubicin | Poly-l-lysine-lipoic acid | Podophyllotoxin | DoxorubicinThe conjugates decreased the hemolytic activity of the drug. The conjugates’ antitumor activity against MCF-7/ADR xenograft tumors was high, with a tumor suppression rate of 82.5%. | The conjugates exhibited enhanced internalization and cytotoxicity effects in vitro. It also exhibited excellent good tumor-targeting capability. | The drug was conjugated into poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) (PLG-g-mPEG) via ester bonds. | [ | It was prepared by the modification of dimethylmaleic anhydride for enhanced cell internalization | 117] | |||||||||||||||||
[ | ] | Polyamidoamine-Pamidronate-Platinum (PAMAM-PAM-Pt) | Polyamidoamine | Pamidronate and platinum | The conjugates were not toxic when compared to the free drugs. | The conjugates were synthesized by aqueous phase Michael-addition polymerization reaction. | [118] | ||||||||||||||||||||
Beta-cyclodextrin- Polyethylene glycol-Folic Acid-doxorubicin (β-CD-PEG-FA-DOX) | Polyethylene glycol, Beta-cyclodextrin | Doxorubicin | Mode of administration in vivo: Intravenous. | Reduced tumor volume, no systemic toxicity, and cardiotoxicity. | Beta-cyclodextrin (β-CD)-based carrier was composed of β-CD, polyethylene glycol, and folic acid for enhanced drug delivery. | [119] | |||||||||||||||||||||
Methoxy Polyethylene glycol-Polylactic acid-Doxorubicin (mPEG-b-PLA-g-DOX) | Polyethylene glycol, Polylactic acid | Doxorubicin | The cytotoxicity studies showed the cytocompatibility of polymeric carriers to MCF-7 breast cancer cell lines with the viability of cells greater than 80%. | The conjugates were prepared by ring-opening polymerization and condensation followed by click reaction. The carriers were grafted with a triazo group. Doxorubicin was modified with cyclooctyne and conjugated to the carriers by strain-promoted alkyne-azide cycloaddition click reaction. | [120] | ||||||||||||||||||||||
poly(oligoethylene glycol acrylate) | (POEG-VBC-DOX) | poly(oligoethylene glycol acrylate) | Doxorubicin | Mode of administration in vivo: Intravenous. | Synergistic anti-tumor and anti-metastasis activity in vitro and in vivo. | DOX was incorporated POEG-VBC backbone. | [121] | ||||||||||||||||||||
N-(1,3-dihydroxypropan-2-yl) methacrylamide-Doxorubicin | N-(1,3-dihydroxypropan-2-yl) methacrylamide | Doxorubicin | Extended blood circulation time with an elimination half time of 9.8 h. High accumulation in the tumors and improved in vivo therapeutic efficacy against 4T1 xenograft tumors compared to the free DOX. Tumor inhibition was via inhibition of cell proliferation and antiangiogenic effects. | The conjugates were synthesized by RAFT polymerization, followed by drug conjugation. | [122] | ||||||||||||||||||||||
Polymalic acid-Trastuzumab | Polymalic acid | Trastuzumab | Mode of administration in vivo: Intravenous. | Enhanced tumor growth inhibition. | Polyethylene glycol (PEG) and poly (β-l-malic acid)-drug conjugates were prepared by covalently incorporating anti-HER2/neu peptide. | [123] | |||||||||||||||||||||
Polyamidoamine-Procaine-Platinum-Alendronate | Polyamidoamine | Procaine, Platinum (II), Alendronate | Selective inhibitory effects of the conjugates towards the cancer cell lines. | The conjugates were synthesized by aqueous phase Michael-addition polymerization reaction. | [124] | ||||||||||||||||||||||
Polyamidoamine-Procaine-Pt-Alendronate | Polyamidoamine | Ferrocene, Pt (II) | Selective inhibitory effects of the conjugates towards the cancer cell lines. | The conjugates were synthesized by aqueous phase Michael-addition polymerization reaction. | [125] |
5. Polymer-Anticancer Drug Conjugates for Lung Cancer Treatment
References
- Sharma, B.; Singh, S.; Kanwar, S.S. L-Methionase: A Therapeutic Enzyme to Treat Malignancies. BioMed Res. Int. 2014, 2014, 13.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667.
- Ferlay, J.; Shin, H.-R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2008, 127, 2893–2917.
- World Health Organization. Latest Global Cancer Data: Cancer Burden Rises to 18.1 Million New Cases and 9.6 Million Cancer Deaths in 2018 Latest Global Cancer Data. Available online: https://www.iarc.fr/featured-news/latest-global-cancer-data-cancer-burden-rises-to-18-1-million-new-cases-and-9-6-million-cancer-deaths-in-2018/ (accessed on 1 November 2019).
- Feng, S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114.
- Kakde, D.; Jain, D.; Shrivastava, V.; Kakde, R.; Patil, A.T. Cancer Therapeutics-Opportunities, Challenges and Advances in Drug Delivery. J. Appl. Pharm. Sci. 2011, 1, 1–10.
- Lu, D.; Lu, T.; Cao, S. Drug Combinations in Cancer Treatments Advances in Pharmacoepidemiology & Drug Safety. Adv. Pharmacoepidemiol. Drug Saf. 2013, 2, 1–3.
- Drbohlavova, J.; Chomoucka, J.; Adam, V.; Ryvolova, M.; Eckschlager, T.; Hubalek, J.; Keizek, R. Nanocarriers for Anticancer Drug-New Trends in Nanomedicine. Curr. Drug Metab. 2013, 14, 547–564.
- Mhlwatika, Z.; Aderibigbe, B.A. Polymeric Nanocarriers for the Delivery of Antimalarials. Molecules 2018, 23, 2527.
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavoids loaded in nanocarries: An opportunity to increase oral bioavailibity and bioefficacy. Food Nutr. Sci. 2014, 5, 1212–1227.
- Cui, J.; Yan, Y.; Such, G.K.; Liang, K.; Ochs, C.J.; Postma, A.; Caruso, F. Immobilization and Intracellular Delivery of an Anticancer Drug Using Mussel-Inspired Polydopamine Capsules. Biomacromolecules 2012, 13, 2225–2228.
- Timin, A.S.; Gould, D.J.; Sukhorukov, G.B. Multi-layer microcapsules: Fresh insights and new applications. Expert Opin. Drug Deliv. 2017, 14, 583–587.
- Aaron, A.; Megan, R.; Veronika, K.; Jun, C.; Jennifer, S.; Mark, B.; Jason, W.; Yuping, B.; Eugenia, K. Ultrasound-Triggered Delivery of Anticancer Therapeutics from MRI-Visible Multilayer Microcapsules. Adv. Ther. 2018, 1800051, 1–16.
- Trushina, D.B.; Akasov, R.A.; Khovankina, A.V.; Borodina, T.N.; Bukreeva, T.V.; Markvicheva, E.A. Doxorubicin-loaded biodegradable capsules: Temperature induced shrinking and study of cytotoxicity in vitro. J. Mol. Liq. 2019, 284, 215–224.
- Amgoth, C.; Dharmapuri, G. Synthesis and Characterization of Polymeric Nanoparticles and Capsules as Payload for Anticancer Drugs and Nanomedicines. Mater. Today Proc. 2016, 3, 3833–3837.
- De Koker, S.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867–2884.
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4.
- Guilin, C.; Xiaomin, Z.; Yidan, C.; Robert, J.L.; Jiangfeng, W.; Ju, Y.; Yingxin, Z.; Cheng, Z.; Kaifeng, W.; Bo, Y. Anticancer activity of polymeric nanoparticles containing linoleic acid-SN38 (LA-SN38) conjugate in a murine model of colorectal cancer. Colloids Surf. B Biointerfaces 2019, 181, 822–829.
- Rania, M.H.; Abdelkader, A.M.; Sherweit, H.E.; Eman, S.M.; Noha, A.G.; Salma, A.; Tarek, E.; Rosaline, A.; Maha, F.; Abdullah, I.E.; et al. Dual stimuli-responsive polypyrrole nanoparticles for anticancer therapy. J. Drug Deliv. Sci. Technol. 2018, 47, 176–180.
- Xi, P.; Jingru, C.; Mengdan, Y.; Jie, W.; Guanghua, H.; Yihua, Y.; Meng, H. Enzyme/pH dual-responsive polymer prodrug nanoparticles based on 10-hydroxycamptothecin-carboxymethylchitosan for enhanced drug stability and anticancer efficacy. Eur. Polym. J. 2019, 117, 372–381.
- Long, X.; Mingying, Z.; Wenxia, G.; Yidi, Y.; Jianfeng, Z.; Yuji, P.; Bin, H. Polymeric nanoparticles responsive to intracellular ROS for anticancer drug delivery. Colloids Surf. B Biointerfaces 2019, 181, 252–260.
- Baksi, R.; Pratap, D.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer e ffi cacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526.
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- Kesharwani, P.; Choudhury, H.; Gopal, J.; Pandey, M.; Gorain, B. Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Prog. Mater. Sci. 2019, 103, 484–508.
- Saluja, V.; Mankoo, A.; Saraogi, G.K.; Tambuwala, M.M.; Mishra, V. Smart dendrimers: Synergizing the targeting of anticancer bioactives. J. Drug Deliv. Sci. Technol. 2019, 52, 15–26.
- Du, X.; Yin, S.; Wang, Y.; Gu, X.; Wang, G.; Li, J. Hyaluronic acid-functionalized half-generation of sectorial dendrimers for anticancer drug delivery and enhanced biocompatibility. Carbohydr. Polym. 2018, 202, 513–522.
- Lyu, Z.; Ding, L.; Huang, A.Y.; Kao, C.; Peng, L. Poly(amidoamine) dendrimers: Covalent and supramolecular synthesis. Mater. Today Chem. 2019, 13, 34–48.
- Farmanzadeh, D.; Ghaderi, M. A computational study of PAMAM dendrimer interaction with trans isomer of picoplatin anticancer drug. J. Mol. Graph. Model. 2018, 80, 1–6.
- Yongle, L.; Xujun, Y.; Xi, Y.; Anqi, C.; Lili, Z.; Gang, Z.; Wenbo, L.; Xiangxuan, H.; Juan, L.; Yang, Z.C. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176.
- Basso, J.; Miranda, A.; Nunes, S.; Cova, T.; Vitorino, C.; Pais, A. Hydrogel-Based Drug Delivery Nanosystems for the Treatment of Brain Tumors. Gels 2018, 4, 62.
- Chang, R.; Tsai, W. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098.
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J. In Situ Forming Polymeric Drug Delivery Systems. Indian J. Pharm. Sci. 2009, 71, 242–251.
- Nadine, R.; Katharina, A.; Qiu, Q.; Chao, D.; Rainer, H.; Zhiyuan, Z.; Kai, L. Reductively cleavable polymer-drug conjugates based on dendritic polyglycerol sulfate and monomethyl auristatin E as anticancer drugs. J. Control. Release 2019, 300, 13–21.
- Liu, Y.; Khan, A.R.; Du, X.; Zhai, Y.; Tan, H.; Zhai, G. Progress in the polymer-paclitaxel conjugate. J. Drug Deliv. Sci. Technol. 2019, 54, 101237.
- Tu, Y.; Zhu, L. Enhancing cancer targeting and anticancer activity by a stimulus-sensitive multifunctional polymer-drug conjugate. J. Control. Release 2015, 212, 94–102.
- Martin, H.; Jan, S.; Martin, S.; David, V.; Karel, U.; Jan, K.; Dana, K. Polymer conjugates of acridine-type anticancer drugs with pH-controlled activation. Bioorg. Med. Chem. 2012, 20, 4056–4063.
- Jieqing, M.; Rongfa, G.; Haitao, S.; Fei, L.; Chaogeng, X.; Mingqi, L.; Tianshu, K. Comparison of anticancer activity between lactoferrin nanoliposome and lactoferrin in Caco-2 cells in vitro. Food Chem. Toxicol. 2013, 59, 72–77.
- Nancy, D.; Samantha, M.; Jason, T.; Cynthia, L.; Vasilios, P.; Elena, G.; Christopher, W.E.; Lia, L.; Walid, S.K.; Omid, G.; et al. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 71–81.
- Emiliana, P.; Emilie, A.; Annarita, F.; Paola, S.; Maria, T.; Massimiliano, G.; Stefania, G.; Igor, C. Quantitative and qualitative effect of gH625 on the nanoliposome-mediated delivery of mitoxantrone anticancer drug to HeLa cells. Int. J. Pharm. 2015, 488, 59–66.
- Sabrina, B.; Barbara, D.; Ilaria, R.; Carolina, C.; Ornella, P.; Stefaan De, S.; Eline, P.; Anna, A.; Gabriele, G. In vitro and ex vivo delivery of tailored siRNA-nanoliposomes for E2F1 silencing as a potential therapy for colorectal cancer. Int. J. Pharm. 2017, 525, 377–387.
- Zucker, D.; Barenholz, Y. Optimization of vincristine–topotecan combination-Paving the way for improved chemotherapy regimens by nanoliposomes. J. Control. Release 2010, 146, 326–333.
- Zucker, D.; Andriyanov, A.V.; Steiner, A.; Raviv, U.; Barenholz, Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: Relating structure and pharmacokinetics to therapeutic efficacy. J. Control. Release 2012, 160, 281–289.
- Goftar, M.K.; Rayeni, N.A.; Rasouli, S. Topoisomerase Inhibitors and Types of Them. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2431–2436.
- Khadka, D.B.; Cho, W.-J. Topoisomerase inhibitors as anticancer agents: A patent update. Expert Opin. Ther. Pat. 2013, 23, 1033–1056.
- Kathiravan, M.K.; Kale, A.N.; Nilewar, S. Discovery and Development of Topoisomerase Inhibitors as Anticancer Agents. Mini-Rev. Med. 2016, 16, 1219–1229.
- Hevener, K.E.; Verstak, T.A.; Lutat, K.E.; Riggsbee, D.L.; Mooney, J.W. Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B 2018, 8, 844–861.
- Kaye, S.B. New antimetabolites in cancer chemotherapy and their clinical impact. Br. J. Cancer 1998, 78, 1–7.
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2017, 83, 255–268.
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Chemother. 2009, 8, 2086–2094.
- Huang, C.; Ju, D.; Chang, C.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. BioMedicine 2017, 7, 12–23.
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245.
- Rasmussen, L.; Arvin, A. Chemotherapy-Induced Immunosuppression. Environ. Health Perspect. 1982, 43, 21–25.
- Genevieve, H.; Shannon, B.; Sarah, H.; Karolina, L.; Mckenna, L.; Nicole, S.; Sibaji, S. Drug Resistance in Cancer: An Overview. Cancers (Basel) 2014, 6, 1769–1792.
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front. Oncol. 2019, 9, 487.
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283.
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2015, 5, 53597–53615.
- Liang, X.-J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods Mol. Biol. 2010, 596, 467–488.
- Pang, X.; Yang, X.; Zhai, G. Polymer-drug conjugates: Recent progress on administration routes. Expert Opin. Drug Deliv. 2014, 11, 75–86.
- Elvira, C.; Gallardo, A.; San Roman, J.; Cifuentes, A. Covalent polymer-drug conjugates. Molecules 2005, 10, 114–125.
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853.
- Kumar, K.V. Targeted delivery of nanomedicines. ISRN Pharmacol. 2012, 2012, 1–9.
- Patil, J.P.; Mahajan, H.S. A review on polymer drug conjugate-what, why and how? Int. J. Pharm. Sci. Res. 2015, 6, 4611–4621.
- Chang, M.; Zhang, F.; Wei, T.; Zuo, T.; Guan, Y.; Lin, G.; Shao, W. Smart linkers in polymer–drug conjugates for tumor-targeted delivery. J. Drug Target. 2016, 24, 475–491.
- Feng, Q.; Tong, R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng. Transl. Med. 2016, 1, 277–296.
- Delplace, V.; Couvreur, P.; Nicolas, J. Recent trends in the design of anticancer polymer prodrug nanocarriers. Polym. Chem. 2014, 5, 1529–1544.
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: Ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899.
- Gao, A.X.; Liao, L.; Johnson, J.A. Synthesis of acid-labile PEG and PEG-doxorubicin-conjugate nanoparticles via Brush-First ROMP. ACS Macro Lett. 2014, 3, 854–857.
- Hu, X.; Hu, J.; Tian, J.; Ge, Z.; Zhang, G.; Luo, K.; Liu, S. Polyprodrug amphiphiles: Hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 2013, 135, 17617–17629.
- Liu, J.; Liu, W.; Weitzhandler, I.; Bhattacharyya, J.; Li, X.; Wang, J.; Qi, Y.; Bhattacharjee, S.; Chilkoti, A. Ring-opening polymerization of prodrugs: A versatile approach to prepare well-defined drug-loaded nanoparticles. Angew. Chem. Int. Ed. Engl. 2015, 54, 1002–1006.
- Wang, M.; Wu, B.; Tucker, J.D.; Lu, P.; Lu, Q. Poly(ester amine) constructed from polyethylenimine and pluronic for gene delivery in vitro and in vivo. Drug Deliv. 2016, 23, 3224–3233.
- Marasini, N.; Haque, S.; Kaminskas, L.M. Polymer-drug conjugates as inhalable drug delivery systems: A review. Curr. Opin. Colloid Interface Sci. 2017, 31, 8–29.
- Alven, S.; Aderibigbe, B.A.; Balogun, M.O.; Matshe, W.M.R.; Ray, S.S. Polymer-drug conjugates containing antimalarial drugs and antibiotics. J. Drug Deliv. Sci. Technol. 2019, 53, 101171.
- Srivastava, A.; Yadav, T.; Sharma, S.; Nayak, A.; Kumari, A. Polymers in Drug Delivery. J. Biosci. Med. 2016, 4, 69–84.
- Sanchis, M.; Canal, J.; Lucas, F.; Vicent, R. Polymer-drug conjugates for novel molecular targets. Nanomedicine 2010, 5, 915–935.
- Pasut, G.; Veronese, F.M. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961.
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnol. 2014, 2014, 1–12.
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305.
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment: Miniperspective. J. Med. Chem. 2017, 61, 5108–5121.
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264.
- Lee, E.S.; Na, K.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 2003, 91, 103–113.
- Mahoney, B.P.; Raghunand, N.; Baggett, B.; Gillies, R.J. Tumor acidity, ion trapping and chemotherapeutics: I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem. Pharmacol. 2003, 66, 1207–1218.
- Garcia-Martin, M.L.; Martinez, G.V.; Raghunand, N.; Sherry, A.D.; Zhang, S.; Gillies, R.J. High resolution pHe imaging of rat glioma using pH-dependent relaxivity. Magn. Reson. Med. 2006, 55, 309–315.
- Cafaggi, S.; Russo, E.; Stefani, R.; Leardi, R.; Caviglioli, G.; Parodi, B.; Bignardi, G.; De Totero, D.; Aiello, C.; Viale, M. Preparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin–alginate complex. J. Control. Release 2007, 121, 110–123.
- Yang, R.; Yang, S.G.; Shim, W.S.; Cui, F.; Cheng, G.; Kim, I.W.; Kim, D.D.; Chung, S.J.; Shim, C.K. Lung-specific delivery of paclitaxel by chitosan-modified PLGA nanoparticles via transient formation of microaggregates. J. Pharm. Sci. 2009, 98, 970–984.
- Chun, C.; Lee, S.M.; Kim, S.Y.; Yang, H.K.; Song, S.C. Thermosensitive poly (organophosphazene)–paclitaxel conjugate gels for antitumor applications. Biomaterials 2009, 30, 2349–2360.
- Xiao, K.; Li, Y.; Luo, J.; Lee, J.S.; Xiao, W.; Gonik, A.M.; Agarwal, R.G.; Lam, K.S. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials 2011, 32, 3435–3446.
- Dellian, M.; Yuan, F.; Trubetskoy, V.S.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumour xenograft: Molecular charge dependence. Br. J. Cancer 2000, 82, 1513–1518.
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schäffler, M.; Takenaka, S.; Möller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416.
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185.
- Seymour, L.W.; Ferry, D.R.; Kerr, D.J.; Rea, D.; Whitlock, M.; Poyner, R.; Boivin, C.; Hesslewood, S.; Twelves, C.; Blackie, R.; et al. Phase II studies of polymer-doxorubicin (PK1, FCE28068) in the treatment of breast, lung and colorectal cancer. Int. J. Oncol. 2009, 34, 1629–1636.
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823.
- Matsumoto, Y.; Nichols, J.W.; Toh, K.; Nomoto, T.; Cabral, H.; Miura, Y.; Christie, R.J.; Yamada, N.; Ogura, T.; Kano, M.R.; et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 2016, 11, 533–538.
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349.
- Sadekar, S.; Ray, A.; Janat-Amsbury, M.; Peterson, C.M.; Ghandehari, H. Comparative biodistribution of PAMAM dendrimers and HPMA copolymers in ovarian-tumor-bearing mice. Biomacromolecules 2010, 12, 88–96.
- Danhauser-Riedl, S.; Hausmann, E.; Schick, H.D.; Bender, R.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.R. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD). Invest. New Drugs 1993, 11, 187–195.
- Brown, T.J.; Alchemia Oncology Pty Limited (Eight Mile Plains, AU), Assignee. Hyaluronan-Chemotherapeutic Agent Formulations for the Treatment of Colon Cancer. U.S. Patent 8,388,993, 5 March 2013.
- Singer, J.W. Paclitaxel poliglumex (XYOTAX™, CT-2103): A macromolecular taxane. J. Control. Release 2005, 109, 120–126.
- Camacho, K.M.; Menegatti, S.; Mitragotri, S. Low-molecular-weight polymer–drug conjugates for synergistic anticancer activity of camptothecin and doxorubicin combinations. Nanomedicine 2016, 11, 1139–1151.
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279.
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77.
- Rajora, A.; Ravishankar, D.; Osborn, H.; Greco, F. Impact of the enhanced permeability and retention (EPR) effect and cathepsins levels on the activity of polymer-drug conjugates. Polymers 2014, 6, 2186–2220.
- Canal, F.; Vicent, M.J.; Pasut, G.; Schiavon, O. Relevance of folic acid/polymer ratio in targeted PEG–epirubicin conjugates. J. Control. Release 2010, 146, 388–399.
- Du, C.; Deng, D.; Shan, L.; Wan, S.; Cao, J.; Tian, J.; Achilefu, S.; Gu, Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials 2013, 34, 3087–3097.
- Bassi, P.F.; Volpe, A.; D’Agostino, D.; Palermo, G.; Renier, D.; Franchini, S.; Rosato, A.; Racioppi, M. Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: Results of a phase I study. J. Urol. 2011, 185, 445–449.
- Kunal, P.; Shubham, R.; Pravat, K.; Ananya, D.; Souravi, B.; Sukhen, D.; Kuladip, J.; Parimal, K. Folic acid conjugated curcumin loaded biopolymeric gum acacia microsphere for triple negative breast cancer therapy in invitro and invivo model. Mater. Sci. Eng. C 2019, 95, 204–216.
- Ikhuoria, E.B.; Bach, C. Introduction to Breast Carcinogenesis Symptoms, Risks Factors, Treatment and Management. Eur. J. Eng. Res. Sci. 2018, 3, 58–66.
- Humberto, P.J.; Marilie, D.G.; Hope, L.E.; Jia, C.; Antonia, M.C.; Alfred, I.N.; Regina, M.S.; Mary, S.W.; Susan, L.T. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890.
- Lee, S. Human serum albumin: A nanomedicine platform targeting breast cancer cells. J. Drug Deliv. Sci. Technol. 2019, 52, 652–659.
- Ito, Y. Chemotherapy and Hormone Therapy for Breast Cancer: Current Status and Perspective. J. Jpn. Med. Assoc. 2002, 45, 424–433.
- Cai, H.; Wang, X.; Zhang, H.; Sun, L.; Pan, D.; Gong, Q.; Gu, Z.; Luo, K. Enzyme-sensitive biodegradable and multifunctional polymeric conjugate as theranostic nanomedicine. Appl. Mater. Today 2018, 11, 207–218.
- Vogus, D.R.; Evans, M.E.; Pusuluri, A.; Barajas, A.; Zhang, M.; Krishnan, V.; Nowak, M.; Menegatti, S.; Helgeson, M.E.; Squires, T.D.; et al. A hyaluronic acid conjugate engineered to synergistically and sequentially deliver gemcitabine and doxorubicin to treat triple negative breast cancer. J. Control. Release 2017, 267, 191–202.
- Kumar, A.; Lale, S.V.; Alex, M.R.A.; Choudhary, V.; Koul, V. Colloids and Surfaces B: Biointerfaces Folic acid and trastuzumab conjugated redox responsive random multiblock copolymeric nanocarriers for breast cancer therapy: In-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2017, 149, 369–378.
- Armiñán, A.; Palomino-Schatzlein, M.; Deladriere, C.; Arroyo-Crespo, J.J.; Vicente-Ruiz, S.; Vicent, M.J.; Pineda-Lucena, A. Metabolomics facilitates the discrimination of the specific anti-cancer effects of free-and polymer-conjugated doxorubicin in breast cancer models. Biomaterials 2018, 162, 144–153.
- Arroyo-crespo, J.J.; Arminan, A.; Charbonnier, D.; Balzano-Nogueira, L.; Huertas-Lopez, F.; Marti, C.; Tarazona, S.; Forteza, J.; Cosena, A.; Vicent, M.J. Tumor microenvironment-targeted poly-L-glutamic acid-based combination conjugate for enhanced triple negative breast cancer treatment. Biomaterials 2018, 186, 8–21.
- Gu, Z.; Gao, D.; Al-Zubaydi, F.; Li, S.; Signh, Y.; Rivera, K.; Halloway, J.; Szekely, Z.; Love, S.; Sinko, P.J. The effect of size and polymer architecture of doxorubicin–poly (ethylene) glycol conjugate nanocarriers on breast duct retention, potency and toxicity. Eur. J. Pharm. Sci. 2018, 121, 118–125.
- Zhou, H.; Lv, S.; Zhang, D.; Deng, M.; Zhang, X.; Tang, Z. A polypeptide based podophyllotoxin conjugate for the treatment of multi drug resistant breast cancer with enhanced efficiency and minimal toxicity. Acta Biomater. 2018, 73, 388–399.
- Ndamase, A.S.; Aderibigbe, B.A.; Sadiku, E.R.; Labuschagne, P.; Lemmer, Y.; Ray, S.S.; Nwamadi, M. Synthesis, characterization and in vitro cytotoxicity evaluation of polyamidoamine conjugate containing pamidronate and platinum drug. J. Drug Deliv. Sci. Technol. 2018, 43, 267–273.
- Hyun, H.; Lee, S.; Lim, W.; Jo, D.; Jung, J.S.; Jo, G.; Kim, S.Y.; Lee, D.-W.; Um, S.; Yang, D.h.; et al. Engineered beta-cyclodextrin-based carrier for targeted doxorubicin delivery in breast cancer therapy in vivo. J. Ind. Eng. Chem. 2019, 70, 145–151.
- He, J.; Wang, W.; Zhou, H.; She, P.; Zhang, P.; Cao, Y.; Zhang, X. A novel pH-sensitive polymeric prodrug was prepared by SPAAC click chemistry for intracellular delivery of doxorubicin and evaluation of its anti-cancer activity in vitro. J. Drug Deliv. Sci. Technol. 2010, 53, 101130.
- Xu, J.; Sun, J.; Ho, P.Y.; Luo, Z.; Ma, W.; Zhao, W.; Rathod, S.B.; Fernandez, C.A.; Venkataramanan, R.; Xie, W.; et al. Creatine based polymer for codelivery of bioengineered MicroRNA and chemodrugs against breast cancer lung metastasis. Biomaterials 2019, 210, 25–40.
- Chen, K.; Cai, H.; Zhang, H.; Zhu, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-responsive polymer-doxorubicin conjugate: Antitumor mechanism and potential as nano-prodrug. Acta Biomater. 2019, 84, 339–355.
- Ding, H.; Gangulum, P.R.; Galstyan, A.; Fox, I.; Patil, R.; Hubbard, P.; Murali, R.; Ljubimova, J.Y.; Holler, E. HER2-positive breast cancer targeting and treatment by a peptide-conjugated mini nanodrug. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 631–639.
- Aderibigbe, B.A.; Naki, T.; Steenkamp, V.; Nwamadi, M.; Ray, S.S.; Balogun, M.O.; Matshe, W.M.R. Physiocochemical and in vitro cytotoxicity evaluation of polymeric drugs for combination cancer therapy. Int. J. Polym. Mater. Polym. Biomater. 2019, 16, 1–16.
- Aderbigbe, B.A.; Mugogodi, A.; Mwamadi, M.; Ray, S.S.; Steenkamp, V.; Balogun, M.O.; Matshe, W.M.R. Polyamidoamine-Drug Conjugates Containing Metal-Based Anticancer Compounds. J. Inorg. Organomet. Polym. Mater. 2019, 16, 1–16.
- Tsoukalas, N.; Aravantinou-Fatorou, E.; Baxevanos, P.; Tolia, M.; Tsapakidis, K.; Galanopoulos, M.; Liontos, M. Kyrigias. Advanced small cell lung cancer (SCLC): New challenges and new expectations. Ann. Transl. Med. 2018, 6, 145.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300.
- Latimer, K.M.; Mott, T.F. Lung Cancer: Diagnosis, Treatment Principles, and Screening. Am. Fam. Physician 2015, 91, 250–257.
- Thun, M.J.; Hanan, L.M.; Adams-Campbell, A.L.; Boffetta, P.; Buring, J.E.; Feskanich, D.; Flanders, W.D.; Jee, S.H.; Katanoda, K.; Kolonel, L.N.; et al. Lung Cancer Occurrence in Never-Smokers: An Analysis of 13 Cohorts and 22 Cancer Registry Studies. PLoS Med. 2008, 5, 1357–1371.
- Collins, L.G.; Haines, C.; Perkel, R.; Enck, R.E. Lung cancer: Diagnosis and management. Am. Fam. Physician 2007, 75, 56–63.
- Kumar, E.; Singh, M.; Meena, J.; Singhvi, P.; Thiyagarajan, D.; Saneja, A.; Panda, A.K. Hyaluronic acid-dihydroartemisinin conjugate: Synthesis, characterization and in vitro evaluation in lung cancer cells. Int. J. Biol. Macromol. 2019, 133, 495–502.
- Chen, Y.; Peng, F.; Song, X.; Wu, J.; Yao, W.; Gao, X. Conjugation of paclitaxel to C-6 hexanediamine-modi fi ed hyaluronic acid for targeted drug delivery to enhance antitumor efficacy. Carbohydr. Polym. 2018, 181, 150–158.
- Wang, W.; Zhao, B.; Meng, X.; She, P.; Zhang, P.; Cao, Y.; Zhang, X. Preparation of dual-drug conjugated polymeric micelles with synergistic anti-cancer efficacy in vitro. J. Drug Deliv. Sci. Technol. 2018, 43, 388–396.
- Sun, H.; Chang, M.Y.Z.; Cheng, W.; Wang, Q.; Commisso, A.; Capeling, M.; Wu, Y.; Cheng, C. Biodegradable zwitterionic sulfobetaine polymer and its conjugate with paclitaxel for sustained drug delivery. Acta Biomater. 2017, 64, 290–300.
- Lou, T.; Magnusson, J.; Preat, V.; Frederick, R.; Alexander, C.; Bosquillon, C.; Vanbever, R. Synthesis and in vitro Evaluation of Polyethlene Glycol-Paclitaxel Conjugates for Lung Cancer Therapy. Pharm. Res. 2016, 33, 1671–1681.
- Shamay, Y.; Golan, M.; Tyomkin, D.; David, A. Assessing the therapeutic efficacy of VEGFR-1-targeted polymer drug conjugates in mouse tumor models. J. Control. Release 2016, 229, 192–199.
- Yang, S.; Wang, Y.; Ren, Z.; Chen, M.; Chen, W.; Zhang, X. Stepwise pH/reduction-responsive polymeric conjugates for enhanced drug delivery to tumor. Mater. Sci. Eng. C 2018, 82, 234–243.
