Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Nora Tang and Version 1 by Krishna Kant.
Biosensors have an important role in the timely and rapid detection of several pathogens of plants, and this could avoid the introduction of exotic pathogens to newer environments. AuNPs have been widely used to label antibodies specific to target pathogens and develop sensitive and selective biosensors.
- plant pathogen biosensing
- plant disease
- forest diseases
- gold nanoparticles
1. Bacterial Pathogen Detection
The detection of bacterial pathogens is possible by capturing either the whole bacteria or by targeting the DNA sequence-specific to the bacteria. Capturing the DNA probe is usually sensitive and specific to the disease. Colloidal AuNPs were used to label single-stranded DNA (ssDNA) probes specific to Acidovorax avenae subspecies citrulli, the bacterial fruit blotch causing pathogen, and a strip-based DNA sensor was constructed to detect the presence of pathogen rapidly and in on-site settings. Herein, the internal transcribed spacer (ITS) region was used to design the pathogen-specific probe. This dipstick method was sensitive enough to detect 4 nM of target DNA qualitatively. Semi-quantitative detection was also possible through analysis of optical density and target DNA concentration. The LOD was found as 0.48 nM [32][1]. In another example of the detection of Pseudomonas syringe pathovars, which causes large scale bacterial diseases in crop plants, AuNPs labelled DNA probes were used in a colourimetric detection of pathogen DNA molecules. Specific primers were designed from conserved N-terminal region of the hrcV gene and probes were then designed with thiol-capping at 5’ or 3’ ends of the probes. The colorimetric detection led to color change from red (non-hybridized AuNP labelled DNA probe) to purple (probe becomes hybridized to target DNA) leading to the identification of pathogen DNA present in the sample [33][2]. A similar strategy was followed for the detection of soil bacterium Ralstonia solanacearum, which causes wilt disease in potatoes. The direct detection of unamplified R. solanacearum DNA occurred in a colorimetric AuNP-probe based assay, where the hybridisation of a specific AuNP bound probe with target DNA prevents any aggregation of the AuNPs in the presence of acidic conditions, and thus the colour of the solution remains red indicating the presence of specific R. solanacearum DNA. In negative samples, there is no hybridization, and hence AuNPs aggregate and colour change to purple can be detected. The nano-biosensor was found to be rapid, sensitive, and specific towards the detection of R. solanacearum directly from soil samples without any DNA amplification steps [34][3].
In this regard, an electrochemical biosensor based on sandwich immunoassay involving the capture antibody, the detection antibody, AuNPs, and enzyme horseradish peroxidase (HRP) was developed for the detection of quarantine bacterial pathogen Pantoea stewartii subspecies stewartii-NCPPB 449 (PSS), which is responsible for causing Stewart’s vascular wilt in maize. The high conductivity and surface area properties of AuNPs was utilised to amplify the electrochemical signal and conjugate the HRP-labelled anti-PSS detection antibody, which was then used to detect the presence of bacterial cells bound to the capture antibody. The current response upon the addition of HRP substrate and its subsequent catalytic activity was measured, and a linear relationship was observed with bacteria concentration. The developed biosensing assay was a limit-of detection of 7.8 × 103 cfu/mL higher than the conventional ELISA, and further showed a non-specificity to other selected pathogens [35][4]. In another study, colloidal gold nanoparticles were used to label monoclonal detection antibodies raised against Pantoea subspecies stewartii-NCPPB 449 in a strip-based immunoassay based on sandwich ELISA. Different dilutions of pathogens were tested to check the sensitivity of the test and the limit of detection was found to be 1 × 105 cfu/mL in standard and spiked samples with no cross reactivity shown with other pathogens [36][5].
Surface-enhanced Raman scattering (SERS) is another useful technique that allows highly sensitive and specific detection of molecules of biological or chemical origin. It has been a widely employed technique in the detection of disease-causing pathogens in plants, animals, and humans as well as food-borne pathogens. Using a combination of SERS based methodology and an isothermal DNA amplification technique. Lau et al. developed a multiplex point-of-care system to achieve the detection of Botrytis cinerea and Pseudomonas syringae along with the fungal pathogen Fusarium oxysporum in tomatoes and Arabidopsis thaliana [37][6]. These pathogens widely affect crop species worldwide and hence the timely and rapid detection is of paramount importance in reducing crop losses. AuNPs were tagged with pathogen-specific Raman reporter as well as DNA capture probe to function as SERS nanotags. The recombinase polymerase amplification (RPA) method was used to amplify pathogen-specific amplicons with a 10 nt 5′ overhang and a biotin label at the other end, being amplified from pathogen genomic DNA isolated from the infected plant. The hybridisation of RPA products with SERS nanotags (the 5′ overhang in RPA amplicons and its complementary AuNP bound DNA probe) was then detected after capturing the biotin-RPA-SERS products with streptavidin tagged magnetic beads and exposing them to laser excitation in a portable Raman spectrometer. Distinct peaks were observed for specific pathogens as per the SERS nanotags and RPA amplicon hybridization and the presence of B. cinerea, P. syringae, and F. oxysporum f.sp conglutinans could be detected. The developed system was sensitive enough to detect B. cinerea DNA as low as two copies and could easily be multiplexed both in the case of tomatoes and Arabidopsis thaliana systems and was successfully demonstrated in outside laboratory settings.
In yet another study, similar RPA based target DNA amplification was coupled with an AuNP based electrochemical sensing platform to sensitive and specifically detect P. syringae, the common plant pathogenic bacterium. In a study by Lau et al., it was found that once the target DNA region was amplified through RPA, it was hybridized with capture DNA probes labelled on to AuNPs and the conjugate was then separated through the binding of biotin tags present in RPA amplicons and streptavidin tagged magnetic beads. The whole complex was heat-treated to denature dsDNA and release AuNPs to bring about the electrochemical reduction of Au+3 to Au0 on a screen-printed carbon electrode and was measured through differential pulse voltammetry (DPV). The more AuNPs are released, the more is the amount of target DNA present in the sample and hence pathogen presence could be detected. The total time to carry out the assay was 60 min and the amplification of target DNA through RPA, as well as the detection limit of the electrochemical assay, were more sensitive (100 times each) when compared to endpoint PCR and gel electrophoresis. Overall, the developed AuNP-electrochemical biosensor was 10,000 times more sensitive and had the capability to detect pathogen presence in the very early stages of infection when tested on P. syringae infected A. thaliana [23][7]. In an attempt to make the lateral flow immunoassay (LFIA) biosensor-based detection of potatoes wilt pathogen R. solanacearum more effective by reducing the limit-of-detection value, wherein the AuNPs undergo gold enhancement [14][8]. Table 21 presents the detection of plant pathogens and various sensing methods, as well as their respective LOD.
Table 21. Different sensing approaches and nanomaterial used for plant pathogen detection.
Different sensing approaches and nanomaterial used for plant pathogen detection.
| No | Plant Disease/Pathogen | Species | Nanomaterial Used | Sensing Method | LOD | Ref. |
|---|---|---|---|---|---|---|
| 1 | Tomato Yellow Leaf Curl Virus (TYLCV) | Tomato | AuNps with colorimetric nano-biosensing | Localized surface plasmon resonance | 5 ng | [9] |
| 2 | Cucumber Mosaic Virus (CMV) and Papaya Ring Spot Virus (PRSV) | Papaya | Nanowire based biosensor | Amperometry detection | 0.1 mA/mL | [10] |
| 3 | Witches’ Broom Disease (Candidatus Phytoplasma aurantifolia) | Lime | Quantum dot (QD)-based nano-biosensor | Fluorescence resonance energy transfer (FRET) | 5 ca. P. aurantifolia/μL | [11] |
| 4 | Odontoglossum Ringspot Virus (ORSV) | Orchid leaves | anodic aluminum oxide (AAO) with AuNPs | Self-assembled monolayer (SAM) | 0.345 ng/mL | [12] |
| 5 | Bacterial Spot Disease by Xanthomonas axonopodis | Solanaceae plant | Fluorescence silica nanoparticles | Fluorescence-linked immunosorbent assay | NA | [13] |
| 6 | Ralstonia solanacearum (Potato Brown Rot) | Potato | Enlarging AuNPs | Lateral flow immunoassay | 3 × 104 cells/mL | [14] |
| 7 | Karnal Bunt Disease | Wheat | AuNPs | Surface Plasmone Resonance (SPR) | NA | [15] |
| 8 | Powdery Mildew | Rose | Colloidal nanosilver (1.5 nm diameter) | Relative fluorescence units | 4.2 μM Ag ions | [16] |
| 9 | Pseudocerocospora fijiensis Black Sigatoka (Leave Streak Disease) | Banana plants | cell wall protein HF1 of P. Fijiensis immobilized onto gold chip | Surface plasmon resonance based immunosensor | 11.7 μg/mL, | [17] |
| 10 | Late Blight in Potatoes and Tomatoes (caused by Phytophthora Infestans) |
Potatoes and Tomatoes | AuNPs | PCR with AuNPs based lateral flow biosensor |
0.1 pg/mL range. | [18] |
| 11. | Acidovorax avenae subsp. citrulli | Fruits | Colloidal gold nanoparticles | Dipstick method | 0.48 nM of DNA | [1] |
2. Detection of Fungal Pathogens
Pathogenic fungal species cause a massive loss in crop quality and yields, posing a threat to the economics of global agricultural sector. It has been reported that approximately 8000 species of fungi and oomycetes are causing diseases in plants and other agricultural crops [47][19]. Fungi can cause diseases at any stages of plant growth either alone or in association with other kinds of phytopathogens under natural environmental conditions [48][20]. The most common diseases caused by pathogenic fungi are anthracnose, blight, canker, damping off, dieback, gall, leaf spot, powdery mildew, rust, root rot, scab, and wilt [49][21]. There are several conventional methods such as polymerase chain reaction (PCR) (nested, multiplex, quantitative, magnetic-capture hybridization PCR techniques) and isothermal amplification methods (LAMP), which have been developed for the detection of fungal species. However, these techniques require highly skilled personnel, tedious protocol, and longer time to obtain the results. Recently, the advent of nanotechnology-based biosensors provide an excellent option for the detection of harmful fungal pathogens. Phytophthora infestans is one of the devastating fungi, a causal agent of late blight in potatoes and tomatoes, and a threat to the global agriculture. Therefore, rapid and early detection P. infestans is an essential step to contain the disease from further spread. Zhan et al. integrated universal primer mediated asymmetric PCR with AuNPs-based LFA for the visual detection of P. infestans [46][18]. Herein, the asymmetric PCR was performed to produce a large amount of ssDNA and then sandwich hybridization was performed in LFA. In the presence of target DNA, sandwich-type hybridization reactions among the AuNP–probe, target DNA, and capture probe on the test line of LFA, and the distinct red visible line was produced due to the accumulation of AuNPs. The quantification of the LFA was achieved by measuring the signal intensity of the red line and the LOD was found to be 0.1 pg/μL. The detailed schematics of the assay are depicted in Figure 31A.
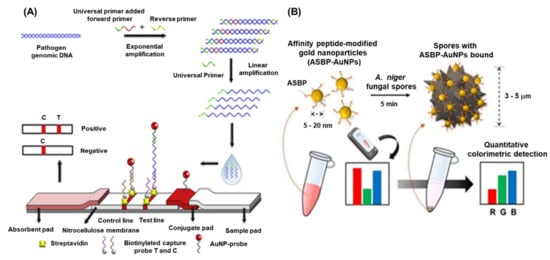
Figure 31. Schematic representation of plant fungi detection by different methods: (A) AuNPs based LFA for the detection of Phytophthora infestans, [46][18] (B) schematic representation of peptide conjugated AuNPs for the detection of A. niger spores. The images are adopted from the references [50][22].
Black sigatoka is a harmful disease caused by the hemi-biotrophic fungus Pseudocercospora fijiensis in banana plantations globally. The detection of this fungal pathogen is important to minimize the losses as well as to prevent the spread to the neighboring cultures. To tackle this pathogen, a highly specific SPR immuno-sensor was developed by Luna –Moreno et al., 2019. The sensor was developed by covalent immobilization of polyclonal antibody (anti-HF; produced against HF1 cell wall protein of P. fijiensis) on Au-coated chip via a mixed self-assembled monolayer (SAM) of alkanethiols [45][17]. The LOD of the SPR immunosensor was found to be 11.7 µg/mL, with a linear response range from 39.1 to 122 µg/mL for the cell wall antigen. The study indicated that there were no matrix effects observed during the analysis of actual leaf banana extracts. Lei et al., 2021, developed an AuNPs-enhanced dynamic microcantilever (MCL) and isothermal recombinase polymerase amplification (RPA) for the detection of Leptosphaeria maculans, a virulent phyto-pathogen of oilseed rape [50][22]. It has been studied that L. maculans produces a phytotoxin, i.e., sirodesmin PL and can sustain for a long period under ambient weather conditions. The detection results showed that the sensitivity of the RPA-MCL assay is higher than that of the previously studied fluorescence RPA assay, with a LOD of one copy of L. maculans DNA. Furthermore, the rapid and sensitive detection of fungal spores is of great interest due to the potential negative impacts on agriculture as well as public health. Lee et al., 2021, developed a technique for the detection of Aspergillus niger spore based on the specific peptides as a recognition probe and the AuNPs as the detection label [51][23]. The peptides enable rapid binding to the A. niger spore resulting in a visible change of colour intensity of the supernatant after sedimentation of the spores. This colorimetric assay displayed a high-sensitivity of −50 spores within <10 min when employed with a smartphone-enabled image analysis application (Figure 31B).
3. Detection of Viruses
Compared to other types of nanomaterials, AuNPs provide an ideal tool for the virus detection due to numerous reasons, which are already described in the previous sections. The ease of synthesis, surface modifications, stability and biocompatibility, and high absorption coefficient leverages their application in detection platform. The intense red colour can be easily visualized and forms stable bio-conjugates with other biological moiety such as DNA, antibodies, and proteins enabling highly sensitive specific sensing of target analytes. Currently, there are several methods available for the detection of plant virus employing the AuNPs. The methods are based on the colorimetric, fluorescence, or electrochemical, etc.
Cucumber green mottle mosaic virus (CGMMV) causes a severe mosaic symptom of watermelon and cucumber, and can be transmitted via infected cucumber seeds, leaves, and soil. Considering the high transmissibility of the viruses, the early detection is extremely important to control its further spread on the crop fields. Wang et al., 2017, developed a simple and sensitive label-free colorimetric detection method for CGMMV using the unmodified AuNPs as a colorimetric probe [52][24]. The principle of the assay lies in the binding of RT-PCR target products of CGMMV and species-specific probes, which results in the change in colour after salt (NaCl) induction. Normally, the species-specific probes attach to the surface of AuNPs and thereby increasing their resistance to NaCl-induced aggregation. The developed method did not need any expensive instruments and was capable to detect 30 pg/μL of CGMMV RNA by the naked eye. The assay provided the specificity of 100% with good reproducibility. The localized surface plasmon resonance (LSPR) is another characteristic property of the AuNPs and currently exploited for the development of colorimetric bio-sensing methods. The LSPR generally depends upon the shape size and the surrounding medium of the AuNPs. Razmi et al., employed the LSPR of unmodified AuNPs to detect the tomato yellow leaf curl virus (TYLCV) genome in infected plants [15][9]. The specific DNA probe that was complimentary against the coat protein of TYLCV was designed and hybridized with the extracted total DNA from the infected sample. The hybridization was performed, cooled to room temperature, and the subsequent addition of the AuNPs indicated the change of colour. The colour change due to the AuNPs suggested the presence of the target virus infection visually and confirmed by the UV-Vis spectroscopy. A similar type of visual colorimetric method has also been developed for Begomovirus in chili and tomato plants with a sensitivity of 500 ag/µL of begomo viral DNA [53][25]. The comparative screening of chili plants for begomoviral infection by PCR and AuNP assay demonstrated that the AuNP assay (77.7%) was better than the commonly used PCR methods (49.4%). More advanced detection methods have been performed using attenuated total reflection (ATR)-based evanescent wave absorption monitoring LSPR of AuNPs [54][26]. The binding dynamics of AuNPs has been studied on the amine-functionalized surface refractive index sensor and was developed by monitoring the LSPR absorption peak. The schematics of the detection platform has been represented in Figure 42A. The method was employed for the detection of single-stranded DNA of the chili leaf curl virus with a LOD of 1.0 μg/mL for target viral DNA.
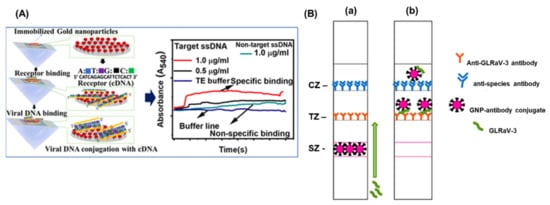
Figure 42. (A) Schematic representation of the detection of Chili Leaf Curl Virus using Attenuated Total Reflection-Mediated Localized Surface-Plasmon-Resonance-Based Optical Platform (B) Lateral Flow Immunoassay for Rapid Detection of Grapevine Leafroll-Associated Virus. (a) is before capturing the GLRaV-3 and (b) after binding of GLRaV-3 with antibody. The images are adopted from the references [54,36][26][5].
The detection of banana bunchy top virus (BBTV) was achieved through an improved AuNPs based dot immunobinding assay (DIBA), which is rapid and simpler than the conventional ELISA [55][27]. Herein, the AuNPs were conjugated to the primary antibody and LOD of the DIBA was found to be at sap dilution of 10−2. Similarly, grapevine leafroll-associated virus 3 (GLRaV-3) is one of the devastating pathogens causing a significant loss in the yield of grapes. For the widespread control of this virus, on-field analytical methods with a high sensitivity are needed. The application of immuno-chromatographic assay (ICA) or lateral flow assay (LFA) for the detection of pathogens and biomarkers have been widely used. The ICA assay employs the AuNPs and provides an easy interpretation of results in the presence or absence of viruses. The key advantages of the ICA are a short analysis time (10–15 min), an ease in sample preparation, and result interpretation. Byzova et al., 2018, developed an ICA for the rapid detection of GLRaV-3 based on the sandwich immunoassay format [36][5]. The researcher compared three preparations of AuNPs, (51.0 ± 7.9 nm, 28.3 ± 3.3 nm, and 18.5 ± 3.3 nm) and showed that AuNPs with maximal average diameters of 51.0 ± 7.9 nm provides GLRaV-3 detection for its maximal dilutions. The assay exhibited a sensitivity of 100% and a specificity of 92% in comparison with ELISA, and a sensitivity of 93% and the specificity of 92% as compared to PCR (Figure 31B). The LFA employing AuNPs sometimes suffers the issues of sensitivity and limit of detection. To enhance the sensitivity, there are several signal enhancement methods that have been developed and applied for the detection of plant viruses. Panferov et al. developed a silver enhancement method for the detection of potato leafroll virus (PLRV) [42][14]. The silver enhancement is based on the reduction of silver ions on the surface of AuNPs and enhances the coloration of AuNPs. This was achieved using a mixture of silver lactate and hydroquinone and the subsequent addition of a chloride-containing buffer to stop the silver reduction. The results suggested that the silver enhanced LFIA was 15 times more sensitive (LOD = 0.2 ng/mL; 15 min) when compared with conventional LFIA (LOD= 3 ng/mL; 10 min). Furthermore, the enhanced LFIA is capable of identifying the detected PLRV in leaves’ extracts of infected potato in dilutions higher than ELISA. Another type of study was performed by Razo et al., 2018, by using magnetic nanoparticles (MNPs) and AuNPs for the double enhancement of the LFA [56][28]. Here, the enrichment of the target was carried out by the specific antibodies conjugated to the magnetic nanoparticles and the signal enhancement was performed by MNPs aggregation through AuNPs. The strategy was employed for the detection of potato virus X (PVX), and the sensitivity of 0.25 ng/mL was achieved and exhibited 32 times more sensitive than the non-enhanced LFA (LOD = 8 ng/mL).
Among various immunoassays, electrochemical immunosensors employing AuNPs enable the development of label-free assays having a shorter analysis time and simplicity over labelled strategies [57,58][29][30]. Khater et al., 2019, developed a label free impedimetric biosensor for the detection of tristeza caused by citrus tristeza virus (CTV). The sensing platform was based on the screen-printed carbon electrode (SPCE) modified by AuNPs. The thiolated ssDNA was immobilized on the surface of AuNPs to enhance the electrode conductivity. The hybridization with the target DNA was investigated by EIS measurements in Fe(CN6)4−/Fe(CN6)3− redox system. The sensor was able to detect the CTV nucleic acids with a linear range of 0.1–10 μM in the presence of other non-specific DNAs [59][31]. Watermelon mosaic virus (WMV) is a major phytopathogen of the family Cucurbitaceae and Leguminosae and induces symptoms of mosaic leaves, decreases of leaf areas, and a umber of fruits, resulting in a serious reduction of yield. Wang et al., 2019, devised a nicking/polymerization strategy for ultrasensitive electrochemical detection of WMV [60][32]. The detection platform is based on the exonuclease and polymerase activity of T4 DNA polymerase and Mg2+-dependent DNAzyme-assisted and hemin/G-quadruplex DNAzyme-assisted cascade amplification strategies. Briefly, the hybridized DNA of the target WMV sequence, i.e., HP1, and P1 was recognized and nicked by nicking endonuclease. The DNA segments were digested in the 3′→5′ direction and was halted at the 3′-terminal G locus with the exonuclease activity of T4 DNA polymerase. The Mg2+-dependent DNAzyme was synthesized by T4 DNA polymerase after the addition of the dNTPs, which hybridized with its substrate sequence immobilized on Au electrode and initiated the cleavage round. Subsequently, the caged G-quadruplex sequence was released and formed hemin/G-quadruplex-based DNAzyme, resulting in the generation of electrochemical signals. The assay showed a linear dynamic range of detection in 50 fM to 1 nM with a LOD of 50 fM.
References
- Zhao, W.; Lu, J.; Ma, W.; Xu, C.; Kuang, H.; Zhu, S. Rapid on-site detection of Acidovorax avenae subsp. citrulli by gold-labeled DNA strip sensor. Biosens. Bioelectron. 2011, 26, 4241–4244.
- Vaseghi, A.; Safaie, N.; Bakhshinejad, B.; Mohsenifar, A.; Sadeghizadeh, M. Detection of Pseudomonas syringae pathovars by thiol-linked DNA–Gold nanoparticle probes. Sens. Actuators B Chem. 2013, 181, 644–651.
- Khaledian, S.; Nikkhah, M.; Shams-bakhsh, M.; Hoseinzadeh, S. A sensitive biosensor based on gold nanoparticles to detect Ralstonia solanacearum in soil. J. Gen. Plant Pathol. 2017, 83, 231–239.
- Zhao, Y.; Liu, L.; Kong, D.; Kuang, H.; Wang, L.; Xu, C. Dual Amplified Electrochemical Immunosensor for Highly Sensitive Detection of Pantoea stewartii sbusp. stewartii. ACS Appl. Mater. Interfaces 2014, 6, 21178–21183.
- Byzova, N.; Vinogradova, S.; Porotikova, E.; Terekhova, U.; Zherdev, A.; Dzantiev, B. Lateral Flow Immunoassay for Rapid Detection of Grapevine Leafroll-Associated Virus. Biosensors 2018, 8, 111.
- Lau, H.Y.; Wang, Y.; Wee, E.J.H.; Botella, J.R.; Trau, M. Field Demonstration of a Multiplexed Point-of-Care Diagnostic Platform for Plant Pathogens. Anal. Chem. 2016, 88, 8074–8081.
- Ebrahimi, M.; Norouzi, P.; Safarnejad, M.R.; Tabaei, O.; Haji-Hashemi, H. Fabrication of a label-free electrochemical immunosensor for direct detection of Candidatus Phytoplasma Aurantifolia. J. Electroanal. Chem. 2019, 851, 113451.
- Lau, H.Y.; Wu, H.; Wee, E.J.H.; Trau, M.; Wang, Y.; Botella, J.R. Specific and Sensitive Isothermal Electrochemical Biosensor for Plant Pathogen DNA Detection with Colloidal Gold Nanoparticles as Probes. Sci. Rep. 2017, 7, 38896.
- Razmi, A.; Golestanipour, A.; Nikkhah, M.; Bagheri, A.; Shamsbakhsh, M.; Malekzadeh-Shafaroudi, S. Localized surface plasmon resonance biosensing of tomato yellow leaf curl virus. J. Virol. Methods 2019, 267, 1–7.
- Ariffin, S.A.B.; Adam, T.; Hashim, U.; Sfaridah, S.F.; Zamri, I.; Uda, M.N.A. Plant Diseases Detection Using Nanowire as Biosensor Transducer. Adv. Mater. Res. 2014, 832, 113–117.
- Detection of Candidatus Phytoplasma Aurantifolia with a Quantum Dots Fret-Based Biosensor on Jstor. Available online: https://www.jstor.org/stable/45156279 (accessed on 26 October 2021).
- Jian, Y.-S.; Lee, C.-H.; Jan, F.-J.; Wang, G.-J. Detection of Odontoglossum Ringspot Virus Infected Phalaenopsis Using a Nano-Structured Biosensor. J. Electrochem. Soc. 2018, 165, H449.
- Yao, K.S.; Li, S.J.; Tzeng, K.C.; Cheng, T.C.; Chang, C.Y.; Chiu, C.Y.; Liao, C.Y.; Hsu, J.J.; Lin, Z.P. Fluorescence Silica Nanoprobe as a Biomarker for Rapid Detection of Plant Pathogens. Adv. Mater. Res. 2009, 79–82, 513–516.
- Razo, S.C.; Panferova, N.A.; Panferov, V.G.; Safenkova, I.V.; Drenova, N.V.; Varitsev, Y.A.; Zherdev, A.V.; Pakina, E.N.; Dzantiev, B.B. Enlargement of Gold Nanoparticles for Sensitive Immunochromatographic Diagnostics of Potato Brown Rot. Sensors 2019, 19, 153.
- Singh, S.; Singh, M.; Agrawal, V.V.; Kumar, A. An attempt to develop surface plasmon resonance based immunosensor for Karnal bunt (Tilletia indica) diagnosis based on the experience of nano-gold based lateral flow immuno-dipstick test. Thin Solid Films 2010, 519, 1156–1159.
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074.
- Luna-Moreno, D.; Sánchez-Álvarez, A.; Islas-Flores, I.; Canto-Canche, B.; Carrillo-Pech, M.; Villarreal-Chiu, J.F.; Rodríguez-Delgado, M. Early Detection of the Fungal Banana Black Sigatoka Pathogen Pseudocercospora fijiensis by an SPR Immunosensor Method. Sensors 2019, 19, 465.
- Zhan, F.; Wang, T.; Iradukunda, L.; Zhan, J. A gold nanoparticle-based lateral flow biosensor for sensitive visual detection of the potato late blight pathogen, Phytophthora infestans. Anal. Chim. Acta 2018, 1036, 153–161.
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. MBio 2020, 11, e00449-20.
- Hariharan, G.; Prasannath, K. Recent Advances in Molecular Diagnostics of Fungal Plant Pathogens: A Mini Review. Front. Cell. Infect. Microbiol. 2021, 10, 829.
- Iqbal, Z.; Khan, M.A.; Sharif, M.; Shah, J.H.; ur Rehman, M.H.; Javed, K. An automated detection and classification of citrus plant diseases using image processing techniques: A review. Comput. Electron. Agric. 2018, 153, 12–32.
- Lei, R.; Wu, P.; Li, L.; Huang, Q.; Wang, J.; Zhang, D.; Li, M.; Chen, N.; Wang, X. Ultrasensitive isothermal detection of a plant pathogen by using a gold nanoparticle-enhanced microcantilever sensor. Sens. Actuators B Chem. 2021, 338, 129874.
- Lee, J.I.; Jang, S.C.; Chung, J.; Choi, W.K.; Hong, C.; Ahn, G.R.; Kim, S.H.; Lee, B.Y.; Chung, W.J. Colorimetric allergenic fungal spore detection using peptide-modified gold nanoparticles. Sens. Actuators B Chem. 2021, 327, 128894.
- Wang, L.; Liu, Z.; Xia, X.; Yang, C.; Huang, J.; Wan, S. Colorimetric detection of Cucumber green mottle mosaic virus using unmodified gold nanoparticles as colorimetric probes. J. Virol. Methods 2017, 243, 113–119.
- Lavanya, R.; Arun, V. Detection of Begomovirus in chilli and tomato plants using functionalized gold nanoparticles. Sci. Rep. 2021, 11, 14203.
- Das, S.; Agarwal, D.K.; Mandal, B.; Rao, V.R.; Kundu, T. Detection of the Chilli Leaf Curl Virus Using an Attenuated Total Reflection-Mediated Localized Surface-Plasmon-Resonance-Based Optical Platform. ACS Omega 2021, 6, 17413–17423.
- Majumder, S.; Johari, S. Development of a gold-nano particle based novel dot immunobinding assay for rapid and sensitive detection of Banana bunchy top virus. J. Virol. Methods 2018, 255, 23–28.
- Razo, S.C.; Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Double-enhanced lateral flow immunoassay for potato virus X based on a combination of magnetic and gold nanoparticles. Anal. Chim. Acta 2018, 1007, 50–60.
- Wang, L.; Shan, J.; Feng, F.; Ma, Z. Novel redox species polyaniline derivative-Au/Pt as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 199. Anal. Chim. Acta 2016, 911, 108–113.
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sens. Actuators B Chem. 2017, 253, 115–123.
- Khater, M.; de la Escosura-Muñiz, A.; Quesada-González, D.; Merkoçi, A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta 2019, 1046, 123–131.
- Wang, Y.; Li, B.; Liu, J.; Zhou, H. T4 DNA polymerase-assisted upgrade of a nicking/polymerization amplification strategy for ultrasensitive electrochemical detection of Watermelon mosaic virus. Anal. Bioanal. Chem. 2019, 411, 2915–2924.
More
