Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Nicolas LOUKA.
Brewers’ spent grains constitute a valuable byproduct of the beer industry. They are characterized by a rich nutritional composition consisting of around 70% lignocellulosic fibrous material, 20% proteins, 10% lipids, in addition to vitamins, minerals, amino acids, and phenolic compounds.
- brewers’ spent grains
- agro-industrial byproducts
- valorization
- pretreatments
- biotechnology
1. Introduction
To overcome BSG aggregation-related issues, and to minimize the waste and pollution arising from the brewing industry, an alternative valorization route through biotechnological processing is highly sought-after. In addition to the nutritional value of the spent grains, their availability throughout the year at a very low cost—equivalent to 40.23 USD per ton of BSG—makes them a favorable raw material for various potential applications [13,14][1][2]. Particularly, microbial fermentation is a promising biovalorization method for this high-volume industrial byproduct [2][3]. For instance, it was reported that this cheap feedstock was used to naturally produce the valuable aromatic compound 2-phenylethanol through microbial fermentation mainly using yeasts, competing with the conventional, environmentally unfriendly, chemical synthesis routes [15][4]. A simplified scheme of the brewing process, BSG constituents, and their uses, is illustrated in Figure 1.
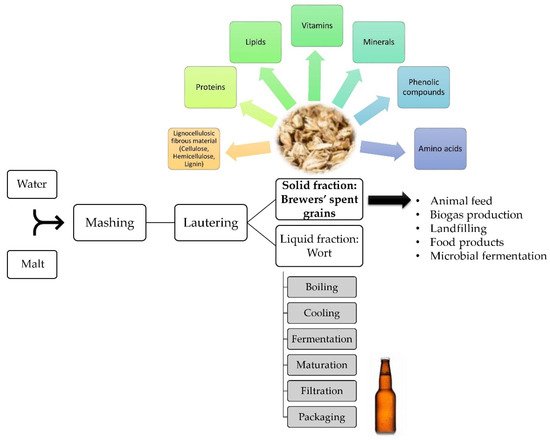
Figure 1. Simplified scheme of the brewing process, focusing on the brewers’ spent grains, their composition, and utilization [2,10,11][3][5][6].
2. Brewers’ Spent Grains Fermentation and Production of Value-Added Compounds
Two types of fermentation might be used in the valorization process of BSG. Both submerged and solid-state fermentation processes will be discussed along with the various value-added products that can be produced via both types of fermentation.
2.1. Submerged Fermentation
2.1.1. Introduction about Submerged Fermentation
Submerged fermentation (SmF) is the classical fermentation type that has been used since the 1940s [42][7]. In this technique, microorganisms grow in a liquid medium containing the needed nutrients. The quantity of water available for bacterial growth affects the development of biomass, metabolic reactions, and mass transfer processes [9][8]. The free-flowing liquid substrates can be either a synthetic media (broths), a waste, or industrial by-product substrate such as molasses. It is noteworthy that this technique is suitable for microorganisms that usually require high moisture content and for the production of secondary metabolites that need to be used in their liquid form since they are usually secreted in the fermentation broth [43][9]. Due to the numerous advantages of this technique, it is being used by many industries and facilities. Particularly, most of the enzyme-producing facilities use SmF due to better monitoring and ease of handling [44][10]. Additionally, this technique allows for the precise control of important growth parameters, including temperature, pH, dissolved oxygen, and substrate concentration [45][11]. SmF is also characterized by its simple downstream processing after fermentation, particularly easy purification of the products [43,46][9][12]. This type of fermentation is less susceptible to substrate inhibition compared to other types, hence leading to a high product yield [47][13]. Scale-up methods and bioreactors for this type of fermentation were well developed; thus, SmF can be used on an industrial scale [48][14]. SmF supports the use of genetically modified organisms [43][9]. On the other hand, however, it has various disadvantages. For one, it is a time-consuming process where secondary metabolites require time to be produced. Also, it is an energy-consuming process, since SmF is prone to bacterial and fungal contamination, and requires energy for sterilization. It is a costly process when synthetic media is used as a substrate and expensive raw materials are added to it. Finally, substrates in SmF are utilized quite rapidly and need to be constantly supplemented with nutrients or replaced [49][15]. Like any other technique, SmF displays advantages and disadvantages, and its usage depends on the field of application and aim of the experiment [42][7].
2.1.2. Valorization of BSG Using Submerged Fermentation
As previously mentioned, BSG can be used as a substrate for the fermentation process and yield various valuable compounds. In this part, the production of various value-added compounds through SmF will be discussed. SmF of BSG using Escherichia coli SL100 can produce ethanol. At first, BSG underwent a phosphoric acid pretreatment in order to recover part of the sugar content (retrieving 80% of the hemicellulose sugar content in BSG and 35.5% of the total glucose in BSG). The liquid fraction from the pretreatment was then fermented using an ethanologenic E. coli SL100, which is a strain capable of converting the spent grains’ xylose, arabinose, and glucose into ethanol and has a high inhibitor tolerance. SmF was carried out with 150 mL of prehydrolysate, for 92 h at pH 6.5, 37 °C, and 300 rpm. After 55 h, a maximal concentration of ethanol was produced which was equal to 16 g/L, corresponding to a yield of 0.4 g of ethanol per g of sugar in the medium [12][16].
Moreover, SmF of BSG using Aspergillus niger and Saccharomyces cerevisiae can produce citric acid. A. niger strain was isolated from spoiled orange and S. cerevisiae strain was isolated from palm wine samples. Fermentation was performed in 500 mL Erlenmeyer flasks containing 200 mL of the fermentation medium and 0.2 mL of methanol (to enhance the fungal production of citric acid) for 14 days at 30 °C and pH 4.5. The highest citric acid concentrations produced were 0.512%/v by A. niger after 96 h fermentation and 0.312%/v by S. cerevisiae after 7 days fermentation [50][17]. Debaryomyces hansenii was also reported to produce ethanol via fermentation on BSG hydrolysates [51][18].
Additionally, SmF of BSG using Lactobacillus rhamnosus can produce L-lactic acid. Fermentation was carried out at 37°C in 300 mL conical flask containing 100 mL immobilized lactic acid bacteria (L. rhamnosus NBRC14710), 70 mL of liquefied BSG that was enzymatically hydrolyzed, and Tween 80 (a growth factor for lactic acid bacteria). After 5 days, L. rhamnosus NBRC14710 produced 19.0 g/L L-lactic acid [52][19].
Furthermore, SmF of BSG using Bacillus sp. KR-8104 can produce α-amylase. Traditionally, SmF is used to produce α-amylase on an industrial scale. The fermentation occurred in 250 mL Erlenmeyer flask containing 50 mL medium (dextrin, yeast extract, meat extract, KH2PO4, and MgSO4·7H2O), 5% (w/v) BSG, and inoculated with a 2% (v/v) bacterial culture at 37 °C and 180 rpm. The maximum production of α-amylase was achieved when using a dextrin-free culture medium and it was around 25,255 U/L (Unit of α-amylase per L of fermented culture medium) [49][15].
In addition, SmF of BSG using Aspergillus fumigatus and Penicillium sp. can produce cellulase. 100 g of BSG were washed with 250 mL of distilled water, at room temperature and 220 rpm for 2 h. Then, the liquid part was recuperated using centrifugation whereas the solid part (washed BSG) was dried at 70°C for 72 h. SmF occurred at 30 °C, 220 rpm, for 96 h in a flask containing 80 g/L washed BSG and culture medium (containing yeast extract, (NH4)NO3, KH2PO4, K2HPO4, and MgSO4) inoculated with a 3-day precultured fungi. The highest cellulase activity was 0.354 and 0.232 U/mL for A. fumigatus and Penicillium sp., respectively [53][20]. Subsequently, various value-added compounds including ethanol, citric acid, L-lactic acid, α-amylase, and cellulase, can be produced through fermentation, particularly SmF, of different microorganisms on BSG and its usage as a carbon and nutrient source for their growth.
2.2. Solid-State Fermentation
2.2.1. Introduction about Solid-State Fermentation
Solid-state fermentation (SSF) is one of the world’s oldest microbiological procedures for fermented food preservation and development. SSF, unlike SmF, is the controlled growth and/or cultivation of microorganisms in the absence of water or with a small amount of water for the manufacture of desired products of interest. The solid matrix can be a source of nutrients or simply support impregnated with the nutrients necessary for microbial development [54,55][21][22]. According to the history of fermentation technology, this method was largely ignored in developed countries after the 1940's at the expense of SmF technology. Following the development of penicillin in SmF and due to the importance of penicillin during the 2nd World War, SmF has become a model technology for the production of any substance via fermentation. As a result, researchers focused solely on the SmF, and the SSF was overlooked. The study of SSF systems has continued in isolated areas, and the transformation of steroids with the help of fungi cultures has been described between the years 1950 and 1960. The trend has continued, albeit slowly, and during the 1960s and 1970s, reports on the production of mycotoxins by the SSF became widely known [56][23]. The second major activity was the production of high-protein animal feed from agricultural waste. Since then, this process has been used extensively, and this trend has accelerated over the last decade [56,57][23][24].
Furthermore, SSF is a well-known and well-established bioprocess for improving nutritional profiles and producing enzymes, particularly used for biomass degradation. The bioreactors used in SSF processes, and classified based on design and operation, are tray bioreactors, packed-bed bioreactors, stirred-drum bioreactors, rotating drum bioreactors, gas-solid fluidized beds, and various stirred-aerated bioreactors [58][25]. Choosing the right bioreactor for the SSF process requires an understanding of the microorganism’s morphology, as well as consideration of the process parameters (e.g., pH, temperature, support type, aeration, moisture, and water activity).
SSF has the potential to be a good bioprocess for the generation of microbial secondary metabolites from agricultural waste and industrial leftovers. Bagasse, bran, husks, whole pomace, seeds, peels, corn residue, BSG, and other waste products are produced in large amounts and are either underutilized or discarded. Recently, there has been a lot of focus on using the abovementioned materials, as they are readily available and low-cost renewable substrates able to generate a variety of valuable chemicals [59][26]. This approach has several advantages over SmF, including lower catabolite repression and substrate inhibition, superior enzyme harvests, environmentally friendly, low output waste, low energy consumption, prolonged product steadiness, no discharge of organic waste water, and low production costs [60,61,62][27][28][29]. In addition, the process is suitable for working with filamentous fungi. It consists of a simple fermentation process with no foam formation and easy control of bacterial contamination [63][30].
Moreover, the disadvantages of this technique depend on the type of bioreactor used. Generally, it is hard to control the metabolite temperature during the process. As a result, the temperature will rise to the point where the intended microbial product will be destroyed or the growth and fermentation are entirely stopped [64][31]. In addition, steady aeration, growth, and kinetic study are still difficult to achieve [63][30].
2.2.2. Valorization of BSG Using Solid State-Fermentation
SSF is a valuable technique for the valorization of agro-industrial byproducts—namely, BSG—to produce value-added products [65][32]. Cellulase and xylanase enzymes can be produced through SSF of A. niger on BSG. SSF was carried out in 250 mL Erlenmeyer flask containing 2 g of BSG in an enriched medium (composed of (NH4)2SO4, NaNO3, KH2PO4, and yeast extract) with a solid to liquid ratio of 3.5:1 (w/v), and an 80% humidity. The medium was then sterilized and inoculated with A. niger CECT 2700 strain acquired from the Spanish collection of type culture. Fermentation occurred at 30 °C for 7 days without mixing. The highest cellulase activity was 6.23 U/g of dry substrate (gds) using BSG pretreated with boiling water, whereas the highest xylanase activity was 1400.80 U/gds obtained using BSG pretreated in an autoclave [66][33]. Another study reported the ability of A. fumigatus and Penicillium sp. to produce cellulase, using SSF. Cellulase was produced by fermenting 5 g of washed BSG inoculated with 4 mL of a 3-day fungi preculture, at 30 °C for 18 days, and with 73.6% humidity. The highest cellulase activity was 7.5 U/gds after 11 days for A. fumigatus, whereas Penicillium sp. produced 8.3 U/gds cellulase after 14 days [53][20].
In addition, SSF of BSG using Penicillium janczewskii can lead to the production of multiple xylanolytic enzymes, particularly xylanase, β-xylosidase and α-L-arabinofuranosidase. SSF was performed in 250 mL Erlenmeyer flasks containing 5 g of dried BSG and Vogel’s salt solution, with a 50% initial moisture, inoculated with 1 mL of P. janczewskii CRM 1348 suspension. SSF was carried out for 7 days at 28 °C. The maximum amounts of xylanase, β-xylosidase, and α-L-arabinofuranosidase produced were 370 U/gds, 246.5 mU/gds, and 606.7 mU/gds, respectively [67][34].
Laccase enzyme can also be produced through the biovalorization of BSG using Trametes versicolor TV-6. 50 g of BSG mixed with 10 mL of distilled water were placed in a flask, autoclaved, and then cooled down. The medium was then inoculated with T. versicolor. SSF occurred for 14 days at 27 °C, with a 63% initial moisture content. The highest laccase activity was produced after 7 days fermentation and it was equal to 560 U/L [68][35].
2-PE is another value-added molecule that can be produced through SSF of BSG. The SSF using Pichia kudriavzevii CECT 13,184 was carried out for 96 h in 500 mL Erlenmeyer flasks containing 95 g of pretreated and inoculated BSG. The highest concentration of 2-PE appeared after 10 h fermentation and at 30 °C and 76% initial content moisture, it was equal to 6.5 mg2−PE/gds in the presence of 4% L-phenylalanine as precursor [69][36].
Ethanol is another alcohol that can be produced through SSF of BSG. SSF of pretreated (by diluted phosphoric acid) and enzymatically hydrolyzed BSG produced ethanol using S. cerevisiae for 72 h at 150 rpm and 40 °C. Actually, after 30 h fermentation, 5.1 g of ethanol were produced for every 100 g of BSG [12][16].
In addition, GA3 can be produced through SSF of BSG. Fermentation occurred in 500 mL flasks containing 50 g BSG in an enriched medium (containing glucose, FeSO4·7H2O, MgSO4, MnSO4·H2O, ZnSO4·7H2O, and at pH 5–5.5). The flasks were then autoclaved and inoculated using Fusarium fujikuroi IOC 4380, at a ratio equal to 15% to the mass of BSG. The highest GA3 production was achieved after 96 h of fermentation, at 28 °C and 80% moisture content, and was equal to 0.82 g per kg of BSG [70][37].
SSF of BSG using Aspergillus oryzae can also produce α-amylase. Fermentation occurred in 250 mL Erlenmeyer flasks containing 5 g of BSG and a salt solution (composed of NH4NO3, KH2PO4, NaCl, and MgSO4·7H2O). The substrate was then autoclaved and inoculated with A. oryzae NRRL 6270. After 96 h fermentation at 30 °C and 70% moisture content, the maximum amount of α-amylase produced by A. niger was 6870 U/gds [71][38].
Finally, as previously discussed, BSG is a cost-effective carbon and nitrogen source that can be used as a substrate for microbial growth [53][20]. Through SSF on BSG, different microorganisms were able to produce various value-added compounds including cellulase, xylanolytic enzymes, laccase, 2-PE, ethanol, GA3, and α-amylase.
References
- Teixeira, M.R.; Guarda, E.C.; Freitas, E.B.; Galinha, C.F.; Duque, A.F.; Reis, M.A.M. Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnol. 2020, 57, 4–10.
- Buffington, J. The Economic Potential of Brewer’s Spent Grain (BSG) as a Biomass Feedstock. Adv. Chem. Eng. Sci. 2014, 4, 308–318.
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052.
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.-M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109.
- Cooray, S.T.; Lee, J.J.L.; Chen, W.N. Evaluation of brewers’ spent grain as a novel media for yeast growth. AMB Express 2017, 7, 1–10.
- San Martin, D.; Orive, M.; Iñarra, B.; Castelo, J.; Estévez, A.; Nazzaro, J.; Iloro, I.; Elortza, F.; Zufía, J. Brewers’ Spent Yeast and Grain Protein Hydrolysates as Second-Generation Feedstuff for Aquaculture Feed. Waste Biomass Valorization 2020, 11, 5307–5320.
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306.
- Chetrariu, A.; Dabija, A. Brewer’s spent grains: Possibilities of valorization, a review. Appl. Sci. 2020, 10, 5619.
- Subramaniyam, R.; Vimala, R. Solid State and Submerged Fermentation for the Production of Bioactive Substances: A Comparative Study. Int. J. Sci. Nat. 2012, 3, 480–486.
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb. Technol. 2010, 46, 541–549.
- Wolters, N.; Schabronath, C.; Schembecker, G.; Merz, J. Efficient conversion of pretreated brewer’s spent grain and wheat bran by submerged cultivation of Hericium erinaceus. Bioresour. Technol. 2016, 222, 123–129.
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Solid-state fermentation strategy for microbial metabolites production: An overview. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Secondary Metabolites Biochemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444635044.
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71.
- Doriya, K.; Jose, N.; Gowda, M.; Kumar, D.S. Solid-State Fermentation vs Submerged Fermentation for the Production of L-Asparaginase. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2016.
- Hashemi, M.; Razavi, S.H.; Shojaosadati, S.A.; Mousavi, S.M. The potential of brewer’s spent grain to improve the production of α-amylase by Bacillus sp. KR-8104 in submerged fermentation system. New Biotechnol. 2011, 28, 165–172.
- Rojas-Chamorro, J.A.; Cara, C.; Romero, I.; Ruiz, E.; Romero-García, J.M.; Mussatto, S.I.; Castro, E. Ethanol Production from Brewers’ Spent Grain Pretreated by Dilute Phosphoric Acid. Energy Fuels 2018, 32, 5226–5233.
- Victor, A.; Titilayo, F.-O. Citric acid production from brewers spent grain by Aspergillus niger and Saccharomyces cerevisiae Enzymes from microorganisms isolated from insect gut View project Citric acid production from brewers spent grain by Aspergillus niger and Saccharomyces cere. Int. J. Res. Biosci. 2013, 2, 30–36.
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of brewery wastes in food industry. PeerJ 2020, 8, e9427.
- Shindo, S.; Tachibana, T. Production of L-lactic acid from spent grain, a by-product of beer production. J. Inst. Brew. 2004, 110, 347–351.
- Casas-Godoy, L.; González-Escobar, J.L.; Mathis, A.G.; Barrera-Martínez, I. Revalorization of untreated Brewer’s spent grain: Novel and versatile feedstock to produce cellulases, lipases, and yeast biomass in a biorefinery approach. Biomass Convers. Biorefinery 2020, 1–12.
- Singh, M.; Devi, S.; Rana, V.S.; Mishra, B.B.; Kumar, J.; Ahluwalia, V. Delivery of phytochemicals by liposome cargos: Recent progress, challenges and opportunities. J. Microencapsul. 2019, 36, 215–235.
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161.
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84.
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30.
- Rodríguez-Couto, S. Solid-State Fermentation for Laccases Production and Their Applications. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–234.
- Lima-Pérez, J.; López-Pérez, M.; Viniegra-González, G.; Loera, O. Solid-state fermentation of Bacillus thuringiensis var kurstaki HD-73 maintains higher biomass and spore yields as compared to submerged fermentation using the same media. Bioprocess Biosyst. Eng. 2019, 42, 1527–1535.
- Krishania, M.; Sindhu, R.; Binod, P.; Ahluwalia, V.; Kumar, V.; Sangwan, R.S.; Pandey, A. Design of Bioreactors in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–96.
- Agarwal, A.K.; Agarwal, R.A.; Gupta, T.; Gurjar, B.R. Biofuels: Technology, Challenges and Prospects. In Green Energy and Technology; Springer: Berlin, Germany, 2017.
- Salgado-Bautista, D.; Volke-Sepúlveda, T.; Figueroa-Martínez, F.; Carrasco-Navarro, U.; Chagolla-López, A.; Favela-Torres, E. Solid-state fermentation increases secretome complexity in Aspergillus brasiliensis. Fungal Biol. 2020, 124, 723–734.
- Hyseni, B.; Aytekin, A.Ö.; Nikerel, E. Solid state fermentation for enzyme production for food industry. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 615–622.
- Costa, J.A.V.; Treichel, H.; Kumar, V.; Pandey, A. Advances in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–17.
- Kumar, A.; Kanwar, S. Lipase production in solid-state fermentation (SSF): Recent developments and biotechnological applications. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2012, 6, 13–27.
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem. Eng. J. 2021, 6, 13–27.
- Terrasan, C.R.F.; Carmona, E.C. Solid-state fermentation of brewer’s spent grain for xylanolytic enzymes production by penicillium janczewskii and analyses of the fermented substrate. Biosci. J. 2015, 31, 1826–1836.
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols. J. Inst. Brew. 2018, 124, 182–186.
- Martínez-Avila, O.; Muñoz-Torrero, P.; Sánchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411.
- da Silva, L.R.I.; de Andrade, C.J.; de Oliveira, D.; Lerin, L.A. Solid-state fermentation in brewer’s spent grains by fusarium fujikuroi for gibberellic acid production. Biointerface Res. Appl. Chem. 2021, 11, 13042–13052.
- Francis, F.; Sabu, A.; Madhavan Nampoothiri, K.; Szakacs, G.; Pandey, A. Synthesis of α-amylase by Aspergillus oryzae in solid-state fermentation. J. Basic Microbiol. 2002, 42, 320–326.
More
