Approaches for effective and sustained drug delivery to the female reproductive tract (FRT) for treating a range of gynaecological conditions remain limited. The development of versatile delivery platforms, such as soluble gels (sol–gels) coupled with applicators/devices, holds considerable therapeutic potential for gynaecological conditions. Sol–gel systems, which undergo solution-to-gel transition, triggered by physiological conditions such as changes in temperature, pH, or ion composition, offer advantages of both solution- and gel-based drug formulations. Furthermore, they have potential to be used as a suitable drug delivery vehicle for other novel drug formulations, including micro- and nano-particulate systems, enabling the delivery of drug molecules of diverse physicochemical character. Hence, such systems are are of profound significance in delivering the drugs to various parts of FRT for optimal treatment of various gynecological conditions which was not achievable using conventional drug delivery technologies.
- vaginal drug delivery
- sol–gel formulations
- stimuli-responsive polymers
- mucoadhesion
- vaginal applicators/devices
1. Introduction
2. Sol–Gel Platform Technology in Vaginal Drug Delivery System
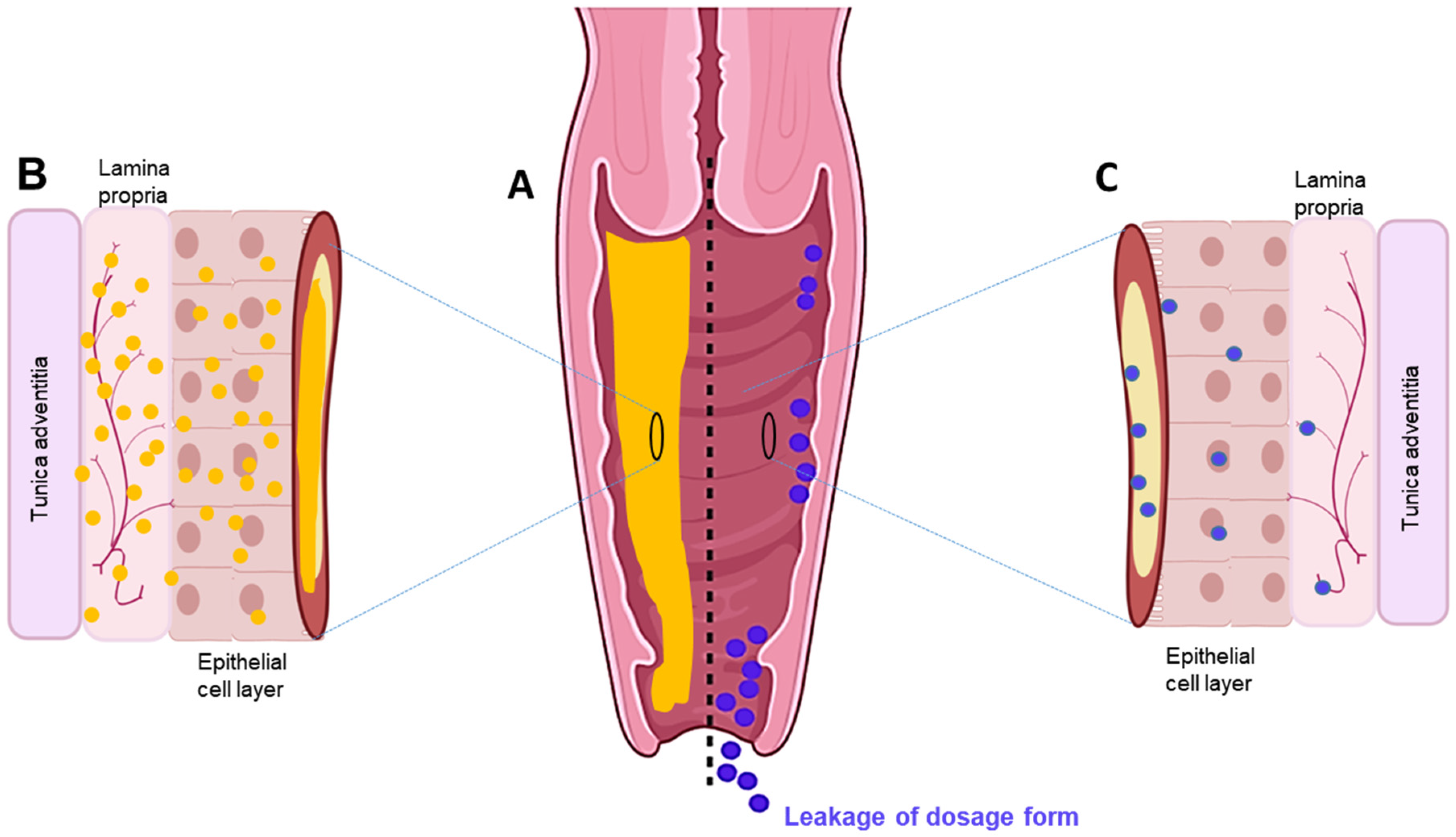 Figure 1. (A) Cross-section of the vaginal tract. (B) Uniform distribution and diffusion of drug throughout mucosal–epithelial layer with sustained delivery using an in situ sol–gel system. (C) Poor and sparse drug distribution through mucosal-epithelial layer and leakage via conventional dosage forms.
Figure 1. (A) Cross-section of the vaginal tract. (B) Uniform distribution and diffusion of drug throughout mucosal–epithelial layer with sustained delivery using an in situ sol–gel system. (C) Poor and sparse drug distribution through mucosal-epithelial layer and leakage via conventional dosage forms.2.1. Features and Use of Vaginal Sol–Gel Formulations
| Active Drug | Brand Name® | Dosage Form | Indication | Manufacturer | Reference |
|---|---|---|---|---|---|
| Oestradiol | Vagifem | Tablet | Atrophic vaginitis | Novo Nordisk Health Care AG | [26] |
| Source | Polymers | Role/Feature | References | ||||
|---|---|---|---|---|---|---|---|
| Plant | Cellulose derivatives e.g., HPMC, HPC, HEC, MC, EC | Thermo responsive gelation; | |||||
| Dinoprostone | Prostin E2, | Tablet | Cervical ripening and labour induction | Pfizer | [27] | ||
| Dinoprostone | Cervidil | Insert | Cervical ripening and labour induction | Forest Laboratories |
[28] | ||
| Misoprostol | Misodel | Insert | Labour induction | Ferring Pharmaceuticals |
[29] | ||
| Mucoadhesive; non-biodegradable | Progesterone | Endometrin | Insert | Assists embryo transplantation |
Ferring Pharmaceuticals |
[30] | |
| Oestradiol | Imvexxy | Inserts | Atrophic vagina | Therapeutics MD | [31] | ||
| Clotrimazole | Gino-Canesten | Cream | Vulovaginal candidiasis | Bayer | [32] | ||
| Sertaconazole | Sertopic | Cream | Vulovaginal candidiasis | ||||
| Pectin | Mucoadhesive | CPH | [ | 32 | ] | ||
| Clindamycin | Dalacin V | Cream | Antibacterial | Pfizer | [32] | ||
| Z. multiflora | Leucorex | Cream | Trichomoniasis | Barijessence | [33] | ||
| Oestriol | Ovestin | Cream | Oestrogen hormone supplement |
Aspen | [32] | ||
| Etonogestrel/ Ethinyloestradiol |
Nuvaring | Ring | Endometriosis, cervical cancer | Organon | [3][34][35][36] | ||
| Progesterone | Progering | Ring | Release progesterone | Laboratorios Andrómaco |
[36] | ||
| Oestradiol | Estring | Ring | Oestrogen replacement therapy, cervical cancer | Pfizer | [3][35] | ||
| Nonoxyl-9 | Today | Sponge | Spermicide | Almatica Pharma, Inc. |
[3] | ||
| Progesterone | Crinone | Gel | Assisted reproductive procedures | Merck | [37] | ||
| Nonoxynol-9 | Vaginal Contraceptive Film | Film | Spermicide | Apothecus | [3] | ||
| Lactobacilli gasser and Lactobacilli rhamnosus | EcoVag | Capsule | Bacterial vaginosis | HÄLSA Pharma GmbH | [38] | ||
| Progesterone | Utrogestran | Capsule | Luteal phase support | Laboratories Besins International | [39] |
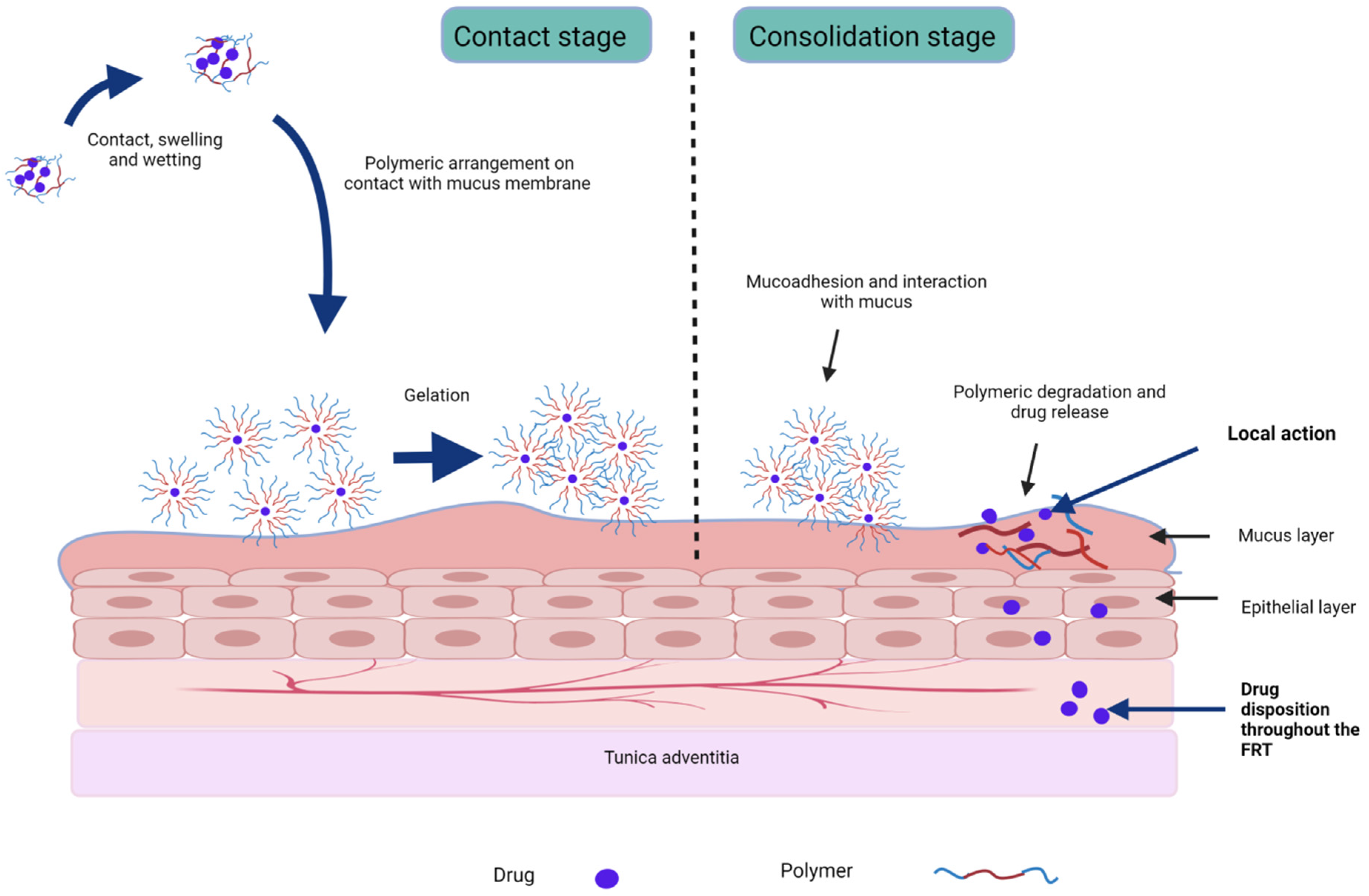 Figure 2. Stages of mucoadhesion and drug release from stimuli-responsive sol–gel formulations.
Figure 2. Stages of mucoadhesion and drug release from stimuli-responsive sol–gel formulations.| Indication | API | Drug Form | Stimuli-Sensitive and Mucoadhesive Polymers (w/v) | Gelation Trigger | Gelation Mechanism |
Comments | References | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial vaginosis | Metronidazole | Free drug | 20% poloxamer 407 and 10% poloxamer 188 | Temperature | Swelling due to polymeric crosslinking | Increased prolonged curative rate with sol–gel (80%) compared to conventional gel (47.4%) | [61] | |||
| Clotrimazole | Free drug | 15% poloxamer 407, 15% and/or 20% poloxamer 188, and 0.2% w/v polycarbophil | Temperature | Micelle formation | Antifungal effect for 10 days; reduced toxicity to epithelium cells of human cervix |
[18][21] | ||||
| Secnidazole | Aerosol foam | 0.45% carbopol 940 with 0.35% HPMC K4 M and 0.35% carbopol 940 with 0.35% HPC | pH | Hydrogen bonding | Less than 50% of drug released by 8 h, indicating controlled drug release | [77] | ||||
| Alginate | Biocompatible; biodegradable; anionic; ion-responsive gelation | Secnidazole | Free drug | |||||||
| Carrageenan | 20% poloxamer 407, 1% poloxamer 188, and 1 or 2.5% chitosan | Temperature | Micelle formation | Mucoadhesive; antimicrobial and antiviral activity | Approximately 1–2-fold increase in mucoadhesiveness with chitosan | [11] | ||||
| [ | 17 | ][85] | Clindamycin | Free drug | 1% gellan gum and 1% HPMC | Ion | Polymeric crosslinking | Good gelling capacity; good mucoadhesion and adequate inhibition of microbial growth | [17][78] | |
| Animal | Chitosan | Polycationic copolymer; Mucoadhesive; biocompatible; biodegradable; antibacterial activity |
[47][17] | Voriconazole | Drug- hydroxypropyl β-cyclodextrin inclusion complex |
Poloxamer 407, poloxamer 188 HPMC, HEC, polycarbophil, and carrageenan | Temperature | Formation of closely packed micelles in aqueous medium | Increased vaginal tissue uptake by the use of cyclodextrin and sustained drug release for 8 h using in situ gel in female Wistar rats compared to conventional formulation |
[60] |
| Gelatin | Biocompatible; biodegradable; | [17] | Amphotericin B | Drug- Hydroxypropyl ϒ-cyclodextrin complex |
25% poloxamer-based multiblock copolymers |
|||||
| Hyaluronic acid | pH and | temperature | Hydrogen bonding | Negatively charged | Toxicity reduced by complexation; dissolution controlled drug release rate; prolonged drug release observed at pH 7.4 and pH 9.0 | [56] | ||||
| [ | 47 | ] | Herpes simplex virus (HSV) infection | Acyclovir | Nanoparticle | 18% poloxamer 407 | pH and temperature | Polymeric crosslinking | Drug’s therapeutic level achieved with 10 times smaller amount of drug; relative bioavailability increased twice compared to suspension dosage form of pure drug |
[58][79] |
| Infertility | Fetilty-Promoting intrauterine infusion liquid (FPL) | Icariin extracted from Epimedium, safflower, and motherwort | 19% poloxamer 407, 2.5% poloxamer 188, and 0.3% HPMC | Temperature | ||||||
| Microbial | Gellan gum | Ion-responsive gelation | [17] | Hydrogen bonding | Uterus and ovarian | indices significantly increased in the rats receiving the sol–gel formulation compared to control group; oestradiol levels increased after day 7 to day 22 |
[80] | |||
| Xanthan gum | Form physical gel | [17] | Sildenafil citrate | Free drug | 15% poloxamer 407 and 1% HEC | Temperature | Entanglement and condensed micelle packing at increased polymer concentration |
Sol–gel transition temperature reduced by addition of HEC; increased endometrial thickness as well as uterine flow with reduced dosing length compared to vaginal suppositories | ||
| Synthetic | Poloxamers | Non-ionic triblock copolymer; amphiphilic; multi-stimuli responsive gelation | [47][17][86 | [ | 81 | ] | ||||
| ] | Pre- exposure prophylaxis of HIV |
Raltegravir + efaviren (RAL + EFV) | ||||||||
| Polyacrylates | Nanoparticles | Viscosity affected by formulation pH | 20% poloxamer 407 and 1% poloxamer 188 | [Temperature | 47]Hydrogen bonding | Inhibitory concentration of RAL + EFV–NPs less than the solution form; sol–gel proved an efficient delivery vehicle of NPs | [13][82] | |||
| Tenofovir | Microsphere | α,β-glycerophosphate (GP), chitosan, sodium alginate | Temperature | Electrostatic interaction between polymers | Viscosity of chitosan–GP complex strengthened by sodium alginate; initial burst release (30%) in the first 30 min followed by cumulative release (87.82%) after 24 hrs | [83] | ||||
| Polyethylene glycol | Water soluble | [17Contraceptive | Nonoxynol-9 | Free drug | 18% poloxamer 407 and 1% or 6% poloxamer | Temperature | Micelle formation |
Increased vaginal residence time compared to solution form; rapid hydrogel erosion and drug release | [11][71] | |
| ] | ||||||||||
| Polyvinylpyrrolidone | Linear; water soluble | [17 | Intrauterine device insertion for contraception |
Lidocaine | Free drug | 18% poloxamer 407, 5% poloxamer 188, and 0.3% gellan gum | Temperature and ionic strength | Hydrogen bonding between the polymers | Better acceptance and pain management by sol–gel formulation compared to conventional gel | [82] |
| ] | Hormone replacement therapy, preterm birth | Progesterone | Free drug | 5% glycol chitin | Temperature | Hydrophobic interaction | No significant effect on gel property by viscosity reduction after dilution by vaginal fluid but not recommended in presence of semen; prolonged vaginal residence time and controlled drug release |
[76][84] | ||
| Cervical cancer | Doxorubicin | Free drug | 7% glycol chitin | Temperature | Hydrophobic interaction | Initial 20% burst release followed by sustained release for 13 days | [75][84] |
2.2. In Situ Sol-to-Gel Phase Transition Stimuli
2.2.1. Thermoresponsive Gelation
Thermosensitive sol–gel systems comprise polymers that undergo gelation at/approaching body temperature. Gelation occurs physically by the entanglement of polymer chains, micelle packing due to self-assembly of the polymeric micelles at elevated temperatures, physical crosslinking due to the dehydration of the polymeric block above a lower critical solution temperature (LCST), hydrophobic interaction, and the transition of a coil into helix form [6][8]. The aqueous solutions of thermogelling polymers have a perfect balance of hydrophilic and hydrophobic groups, which is disturbed with the slightest change in temperature, and they undergo phase separation at the critical solution temperature (CST) [8][21]. For the polymers exhibiting LCST, phase separation occurs above CST, whie the opposite is true for polymers exhibiting an upper critical solution temperature (UCST) [87]. The hydrophilic polymers become hydrophobic and insoluble above their LCST, resulting in gel formation (Figure 3A). LCST determines the thermo-reversibility of thermoresponsive systems and depends upon the polymer concentration [8]. There is an inverse relationship between polymer concentration and gelation temperature, driven by the hydrophobic force [8][88]. At a higher polymer concentration, hydrophobic interaction increases due to molecular crowding, resulting in gelation at a lower temperature [79]. Polymers are typically used in concentrations that trigger gelation in the 25–37 °C range, in the context of VDD. Using an appropriate applicator, an appropriately engineered sol–gel can provide ease of application, while its rapid transformation to a viscous gel can reduce leakage, enhancing retention on the vaginal mucosa [6]. Here, a gelling temperature close to the physiological temperature is ideal for the stabilisation, solubilisation, and controlled release of hydrophobic drugs, as the polymeric monomers aggregate to form micelles within their hydrophobic core, wherein the solubilised hydrophobic drug resides [21][25]. The concentration of thermogelling polymers, co-solutes, and dilution by fluid in the vagina affect the gelation temperature and the viscosity of the gel formed. Hence, it is essential to characterise thermosensitive systems in simulated conditions to help predict their in vivo performance [8][18]. Temperature-stimulated sol–gel transition is a commonly employed phenomenon in several studies of VDDS, even though dual stimuli are also employed for sol–gel transition (see examples in Table 2).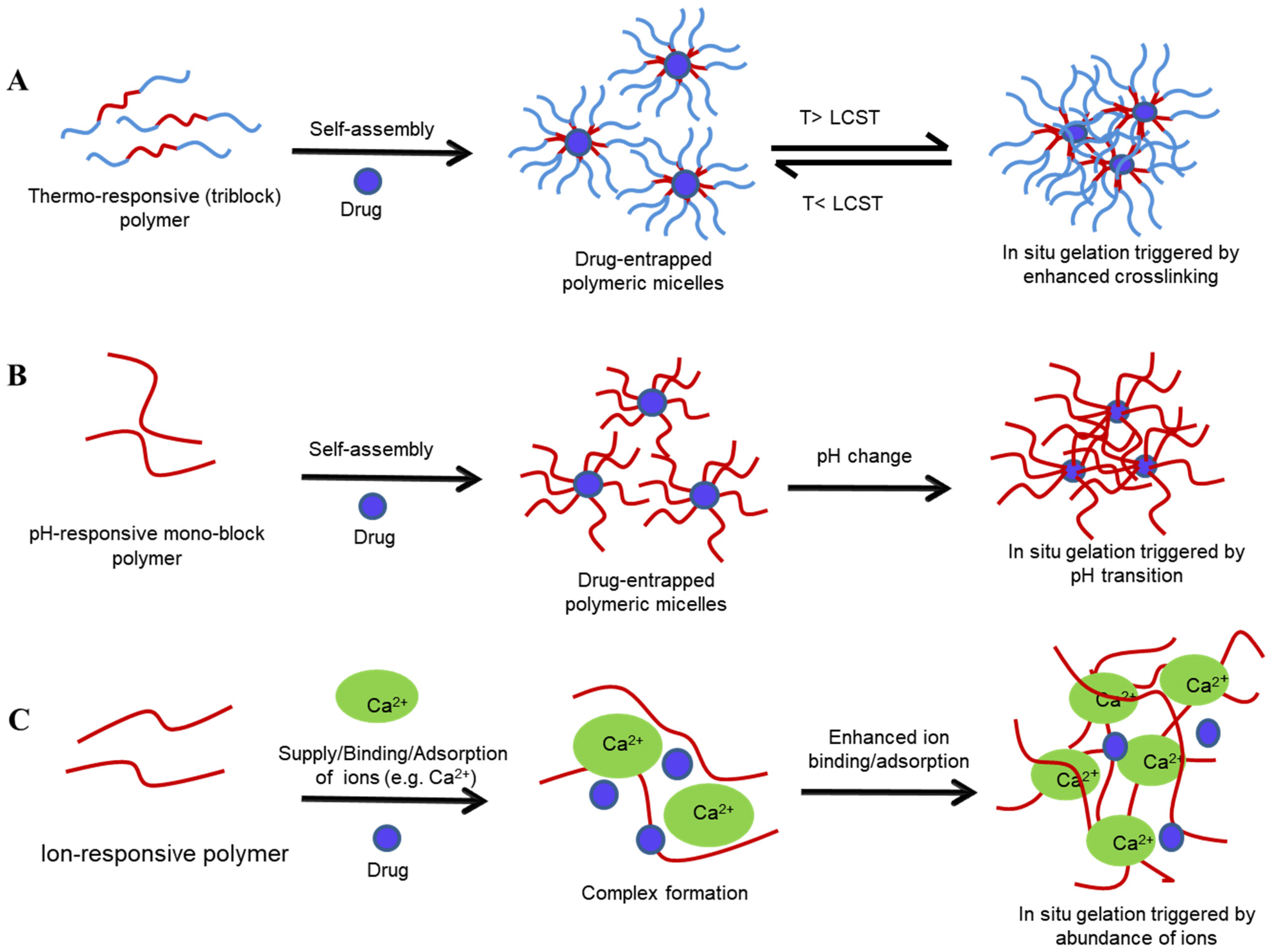 Figure 3. Sol–gel transition of various stimuli-sensitive polymeric systems: temperature-sensitive (A), pH-sensitive (B), and ion-sensitive (C) systems. T—transition temperature, LCST—lower critical solution temperature.
Figure 3. Sol–gel transition of various stimuli-sensitive polymeric systems: temperature-sensitive (A), pH-sensitive (B), and ion-sensitive (C) systems. T—transition temperature, LCST—lower critical solution temperature.
-
Poloxamers
-
Cellulose derivatives
-
Gelatin
2.2.2. pH Sensitive Sol–Gel Systems
Here, polymers contain weakly acidic or basic groups capable of donating or accepting H+ ions depending upon the environmental pH, leading to the ionisation, association, and binding of ions to the polymer chains, resulting in changes in polymer conformation and solubility, both of which are drivers of gelation (Figure 3B) [22][94]. Such changes occur at a specific pH known as the transition/critical pH and it depends upon the pKa of the polymer [1][22]. pH-responsive delivery is a promising approach for the delivery of poorly water-soluble drugs such as paclitaxel, for the treatment of ovarian and cervical cancer. Here, the elevated pH of tumour cells triggers the release of chemotherapeutic agents from the drug formulation containing the pH-responsive polymer mPEG2000-Isopropylideneglycerol [95]. pH-responsive gelation has also been employed in the prophylaxis of STIs and HIV, wherein drug activity is delayed by the vaginal pH and only triggered in the presence of a higher pH once semen is detected [22]. Human semen, with a pH of 6.5 to 7.0, has a high buffering capacity and hence acts a trigger for gelation and drug release, resulting in the inactivation of HIV or other pathogens. The resulting gel acts as a protective microbicide, coating virus particles at the vaginal epithelium, although a short mucosal residence time usually warrants co-formulation of the gel with mucoadhesive polymers [14][22].-
Chitosan
-
Polyacrylates (PA)
2.2.3. Ion-Sensitive Sol–Gel Systems
Anionic polysaccharides, which undergo gelation by crosslinking in the presence of ions, are employed to create ion-sensitive systems [1]. Here, the solution forms of drug–polysaccharide complexes undergo gelation in the presence of ions existing in vaginal fluid, most typically sodium (Na+), calcium (Ca2+), potassium (K+), and chloride (Cl¯) [2]. Although limited studies have been published that use ion-responsive systems, they provide another avenue for investigation to circumvent the shortfalls related to conventional formulations for VDD.-
Gellan gum
| Disadvantages | Product Examples | Reference | ||||
|---|---|---|---|---|---|---|
| Single use | 114 × 12.7 with a tapered, rounded tip | Comprises plunger, barrel, and cap fabricated from PP and a piston inside the barrel made of non-latex rubber; pre-filled or manual filling |
Reduced cost due to bulk production | Higher plastic waste | KY-gel; Canesten® cream |
[109][111][112] |
| Multiple use | 114.5 × 11.3 | Comprises barrel and plunger fabricated from PE | Can be refilled and reusable, reducing packaging, storage, and transportation costs | Sanitary concerns | Ovestin® intravaginal cream | [106][111] |
-
Alginate
-
Pectin
3. Applicators for Intravaginal Administration of Dosage Forms
The effectiveness of VDDSs is largely influenced by the patients’ acceptance and adherence to treatment regimens, which is ultimately determined by the overall user experience. Acceptable user experience can be achieved by ensuring ease of use and patient comfort when administering any vaginal product [106]. Applicators make the vaginal drug administration convenient and drug delivery more reliable. They are classified as class I medical devices and hence possess low risk to the user and are subjected to minimal regulatory control [107]. Although vaginal products can be administered without an applicator, studies suggest the preference of an applicator, despite the associated elevated costs to patients/consumers. Moreover, the physical attributes of the applicator, including the length, width, colour, comfort, ease of grip and use, overall appearance, and environmental friendliness, have been found to influence the choice of applicator [106][107]. Generally, applicators are an optional tool for administering solid dosage forms such as tablets and capsules. However, their use becomes critical when administering liquids, semi-solids, and foams, which typically require deep insertion of the formulation, and applicators offer the advantage of more uniform drug distribution and localised targeted delivery while mitigating leakage and systemic effect [3][108]. Semi-solid formulations, such as creams and sol–gels, need to be sufficiently free-flowing to be used in syringe applicator-based devices, so that the formulation can be ejected via a plunger with ease [28]. Historically, vaginal applicators were developed to deliver contraceptives to the cervix and hence drug exposure to the entire vaginal mucosal tissue was not considered critical [109]. However, increasingly so, the focus has shifted more towards the development of vaginal microbicides, wherein the applicator’s role has become more critical in ensuring delivery to a larger proportion of the lower FRT [109]. As a result, device manufacturers have designed applicators with pores along their length, which ensures that the formulation spreads across a larger surface area of the vaginal mucosa when actuated; this is in contrast to the delivery profile of conventional applicators that aim to deliver the drug into the cervix and upper FRT [6][109]. Recently, a non-hormonal contraceptive with a pre-filled applicator and multiple-pore design was approved by the U.S. FDA, providing on-demand contraception when used 1 h before or immediately after sexual intercourse [110]. Similarly, dinoprostone is used to induce labour and is administered deep in the endocervical canal using an applicator inserted intravaginally by trained personnel [108]. The lack of a suitably designed applicator can seriously hamper the effectiveness of even the best therapeutics, and so patient experience/acceptability must go hand in hand with dosage form and applicator design if expected clinical outcomes are to be met [100]. Selection of a suitable applicator design for VDD has historically been somewhat of an afterthought, although the tide is turning with new vaginal applicators on the horizon, some of which are highlighted in Table 4.| Applicator Type | Dimensions (mm) | Features | Advantages | |||
|---|---|---|---|---|---|---|
| [ | ||||||
| 112 | ] | |||||
| Single-use squeeze tube | 105 × 29 tube, plus 5-mm-wide applicator tip | Single-piece device fabricated from PE | Pre-filled, cost-effective | Cannot be filled manually |
Norden-Pac applicator |
[111] |
| Multiple pores | - | Presence of PE-fabricated membrane around the reservoir, infused with drug product and with perforations | Covers entire vaginal mucosa immediately after application; uniform drug delivery; pre-filled; biodegradable | High manufacturing cost | Universal vaginal applicator | [109] |
References
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent Advances in the Development of In Situ Gelling Drug Delivery Systems for Non-Parenteral Administration Routes. Pharmaceutics 2020, 12, 859.
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2021, 13, 26.
- Neves, J.D.; de Oliveira, R.P.; de Oliveira, A.P.; Rodrigues, F.; Sarmento, B. Vaginal mucosa and drug delivery. In Mucoadhesive Materials and Drug Delivery Systems, 1st ed.; Wiley: Chichester, UK, 2014; pp. 99–132.
- Wong, T.W.; Dhanawat, M.; Rathbone, M.J. Vaginal drug delivery: Strategies and concerns in polymeric nanoparticle development. Expert Opin. Drug Deliv. 2014, 11, 1419–1434.
- Mirza, M.A.; Panda, A.K.; Asif, S.; Verma, D.; Talegaonkar, S.; Manzoor, N.; Khan, A.; Ahmed, F.J.; Dudeja, M.; Iqbal, Z. A vaginal drug delivery model. Drug Deliv. 2016, 23, 3123–3134.
- Cook, M.T.; Brown, M.B. Polymeric gels for intravaginal drug delivery. J. Control. Release 2018, 270, 145–157.
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52.
- Matanović, M.R.; Kristl, J.; Grabnar, P.A. Thermoresponsive polymers: Insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int. J. Pharm. 2014, 472, 262–275.
- Alexander, A.A.; Khan, J.; Giri, T.K.; Tripathi, D.K.; Saraf, S.; Saraf, S. Advancement in stimuli triggered in situ gelling delivery for local and systemic route. Expert Opin. Drug Deliver. 2012, 9, 1573–1592.
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158.
- Argenta, D.F.; Bernardo, B.D.C.; Chamorro, A.F.; Matos, P.R.; Caon, T. Thermosensitive hydrogels for vaginal delivery of secnidazole as an approach to overcome the systemic side-effects of oral preparations. Eur. J. Pharm. Sci. 2021, 159, 105722.
- Mennini, N.; Casella, G.; Cirri, M.; Maestrelli, F.; Mura, P. Development of cyclodextrin hydrogels for vaginal delivery of dehydroepiandrosterone. J. Pharm. Pharmacol. 2016, 68, 762–771.
- Date, A.A.; Shibata, A.; Goede, M.; Sanford, B.; la Bruzzo, K.; Belshan, M.; Destache, C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+ efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral Res. 2012, 96, 430–436.
- Antimisiaris, S.G.; Mourtas, S. Recent advances on anti-HIV vaginal delivery systems development. Adv. Drug Deliv. Rev. 2015, 92, 123–145.
- Dorr, M.L.; Pierson, R.C.; Daggy, J.; Quinney, S.K.; Haas, D.M. Buccal versus vaginal misoprostol for term induction of labor: A retrospective cohort study. Am. J. Perinatol. 2019, 36, 765.
- Tosti, C.; Biscione, A.; Morgante, G.; Bifulco, G.; Luisi, S.; Petraglia, F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 61–66.
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J. Recent Advances in Polymer-Based Vaginal Drug Delivery Systems. Pharmaceutics 2021, 13, 884.
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122.
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162.
- Taurin, S.; Almomen, A.A.; Pollak, T.; Kim, S.J.; Maxwell, J.; Peterson, C.M.; Owen, S.C.; Janát-Amsbury, M.M. Thermosensitive hydrogels a versatile concept adapted to vaginal drug delivery. J. Drug Target. 2018, 26, 533–550.
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.G.M.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265.
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63.
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016.
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426.
- da Silva, J.B.; Cook, M.T.; Bruschi, M.L. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: Mechanical, rheological and sol-gel transition analysis. Carbohydr. Polym. 2020, 240, 116268.
- Pickar, J.; Amadio, J.; Bernick, B.; Mirkin, S. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric 2016, 19, 181–187.
- Abdelaziz, A.; Mahmoud, A.A.; Ellaithy, M.I.; Abees, S.H. Pre-induction cervical ripening using two different dinoprostone vaginal preparations: A randomized clinical trial of tablets and slow release retrievable insert. Taiwan. J. Obstet. Gynecol. 2018, 57, 560–566.
- Bernkop-Schnürch, A.; Hornof, M. Intravaginal Drug Delivery Systems: Design, Challenges, and Solutions. Am. J. Adv. Drug Deliv. 2003, 1, 241–254.
- Bolla, D.; Weissleder, S.V.; Radan, A.P.; Gasparri, M.L.; Raio, L.; Müller, M.; Surbek, D. Misoprostol vaginal insert versus misoprostol vaginal tablets for the induction of labour: A cohort study. BMC Pregnancy Childbirth 2018, 18, 149.
- Peet, M.M.; Agrahari, V.; Anderson, S.M.; Hanif, H.; Singh, O.N.; Thurman, A.R.; Doncel, G.F.; Clark, M.R. Topical Inserts: A Versatile Delivery Form for HIV Prevention. Pharmaceutics 2019, 11, 374.
- Liu, J.H.; Bernick, B.; Mirkin, S. Estradiol softgel inserts for the treatment of VVA symptoms: An expert opinion. Expert Opin. Drug Deliv. 2020, 17, 1573–1581.
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568.
- Küng, E.; Fürnkranz, U.; Walochnik, J. Chemotherapeutic options for the treatment of human trichomoniasis. Int. J. Antimicrob. Agents 2019, 53, 116–127.
- Friend, D.R. Drug delivery for the treatment of endometriosis and uterine fibroids. Drug Deliv. Transl. Res. 2017, 7, 829–839.
- Wang, X.; Liu, S.; Guan, Y.; Ding, J.; Ma, C.; Xie, Z. Vaginal drug delivery approaches for localized management of cervical cancer. Adv. Drug Deliv. Rev. 2021, 174, 114–126.
- Brache, V.; Faundes, A. Contraceptive vaginal rings: A review. Contraception 2010, 82, 418–427.
- Velázquez, N.S.; Turino, L.N.; Luna, J.A.; Mengatto, L.N. Progesterone loaded thermosensitive hydrogel for vaginal application: Formulation and in vitro comparison with commercial product. Saudi Pharm. J. 2019, 27, 1096–1106.
- Marcotte, H.; Krogh Andersen, K.; Lin, Y.; Zuo, F.; Zeng, Z.; Larsson, P.G.; Brandsborg, E.; Brønstad, G.; Hammarström, L. Characterization and complete genome sequences of L. rhamnosus DSM 14870 and L. gasseri DSM 14869 contained in the EcoVag® probiotic vaginal capsules. Microbiol. Res. 2017, 205, 88–98.
- Child, T.; Leonard, S.A.; Evans, J.S.; Lass, A. Systematic review of the clinical efficacy of vaginal progesterone for luteal phase support in assisted reproductive technology cycles. Reprod. Biomed. Online 2018, 36, 630–645.
- Sarwal, A.; Singh, G.; Singh, S.; Singh, K.; Sinha, V. Novel and effectual delivery of an antifungal agent for the treatment of persistent vulvovaginal candidiasis. J. Pharm. Investig. 2019, 49, 135–147.
- Enggi, C.K.; Isa, H.T.; Sulistiawati, S.; Ardika, K.A.R.; Wijaya, S.; Asri, R.M.; Mardikasari, S.A.; Donnelly, R.F.; Permana, A.D. Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: A proof of concept study. Int. J. Pharm. 2021, 609, 121182.
- Vigani, B.; Faccendini, A.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Grisoli, P.; Ferrari, F. Development of a mucoadhesive in situ gelling formulation for the delivery of Lactobacillus gasseri into vaginal cavity. Pharmaceutics 2019, 11, 511.
- Choi, S.G.; Lee, S.-E.; Kang, B.-S.; Ng, C.L.; Davaa, E.; Park, J.-S. Thermosensitive and mucoadhesive sol-gel composites of paclitaxel/dimethyl-β-cyclodextrin for buccal delivery. PLoS ONE 2014, 9, e109090.
- Smoleński, M.; Karolewicz, B.; Gołkowska, A.M.; Nartowski, K.P.; Małolepsza-Jarmołowska, K. Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels. Int. J. Mol. Sci. 2021, 22, 6455.
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919.
- Zierden, H.C.; Josyula, A.; Shapiro, R.L.; Hsueh, H.T.; Hanes, J.; Ensign, L.M. Avoiding a Sticky Situation: Bypassing the Mucus Barrier for Improved Local Drug Delivery. Trends Mol. Med. 2021, 27, 436–450.
- Lalan, M.S.; Patel, V.N.; Misra, A. Polymers in vaginal drug delivery: Recent advancements. In Applications of Polymers in Drug Delivery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–303.
- Devadasu, V.R.; Deb, P.K.; Maheshwari, R.; Sharma, P.; Tekade, R.K. Physicochemical, pharmaceutical, and biological considerations in GIT absorption of drugs. In Dosage Form Design Considerations; Academic Press: Cambridge, MA, USA, 2018; pp. 149–178.
- Lacey, C.; Woodhall, S.; Qi, Z.; Sawant, S.; Cowen, M.; McCormack, S.; Jiang, S. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int. J. STD AIDS 2010, 21, 714–717.
- Liu, C.; Jiang, X.; Gan, Y.; Yu, M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: Design, evaluation and state-of-the-art. Med. Drug Discov. 2021, 12, 100110.
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801.
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomed. Nanotechnol. Biol. Med. 2021, 40, 102494.
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41.
- El-Hammadi, M.M.; Arias, J.L. Nanomedicine for vaginal drug delivery. In Theory and Applications of Nonparenteral Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–257.
- El-Hammadi, M.M.; Arias, J.L. Nanotechnology for vaginal drug delivery and targeting. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 647–682.
- Kim, Y.-T.; Shin, B.-K.; Garripelli, V.K.; Kim, J.-K.; Davaa, E.; Jo, S.; Park, J.-S. A thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin B. Eur. J. Pharm. Sci. 2010, 41, 399–406.
- Chindamo, G.; Sapino, S.; Peira, E.; Chirio, D.; Gallarate, M. Recent Advances in Nanosystems and Strategies for Vaginal Delivery of Antimicrobials. Nanomaterials 2021, 11, 311.
- Ramyadevi, D.; Rajan, K.S.; Vedhahari, B.N.; Ruckmani, K.; Subramanian, N. Heterogeneous polymer composite nanoparticles loaded in situ gel for controlled release intra-vaginal therapy of genital herpes. Colloids Surf. B 2016, 146, 260–270.
- Iqbal, R.; Qureshi, O.S.; Yousaf, A.M.; Raza, S.A.; Sarwar, H.S.; Shahnaz, G.; Saleem, U.; Sohail, M.F. Enhanced solubility and biopharmaceutical performance of atorvastatin and metformin via electrospun polyvinylpyrrolidone-hyaluronic acid composite nanoparticles. Eur. J. Pharm. Sci. 2021, 161, 105817.
- Deshkar, S.S.; Palve, V.K. Formulation and development of thermosensitive cyclodextrin-based in situ gel of voriconazole for vaginal delivery. J. Drug Deliv. Sci. Technol. 2019, 49, 277–285.
- Shaaban, O.M.; Fetih, G.N.; Abdellah, N.H.; Ismail, S.; Ibrahim, M.A.; Ibrahim, E.S.A. Pilot randomized trial for treatment of bacterial vaginosis using in situ forming metronidazole vaginal gel. J. Obstet. Gynaecol. Res. 2011, 37, 874–881.
- Jalalvandi, E.; Jafari, H.; Amorim, C.A.; Petri, D.F.S.; Nie, L.; Shavandi, A. Vaginal Administration of Contraceptives. Sci. Pharm. 2021, 89, 3.
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828.
- Traore, Y.L.; Chen, Y.; Ho, E.A. Current state of microbicide development. Clin. Pharmacol. Ther. 2018, 104, 1074–1081.
- Shi, S.; Nguyen, P.K.; Cabral, H.J.; Diez-Barroso, R.; Derry, P.J.; Kanahara, S.M.; Kumar, V.A. Development of peptide inhibitors of HIV transmission. Bioact. Mater. 2016, 1, 109–121.
- Mahalingam, A.; Jay, J.I.; Langheinrich, K.; Shukair, S.; McRaven, M.D.; Rohan, L.C.; Herold, B.C.; Hope, T.J.; Kiser, P.F. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials 2011, 32, 8343–8355.
- Shaikh, R.P.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Ndesendo, V.M.; Bawa, P.; Cooppan, S. A review of multi-responsive membranous systems for rate-modulated drug delivery. AAPS PharmSciTech 2010, 11, 441–459.
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM dendrimer–PEG hydrogels for the treatment of genital infections: Formulation and in vitro and in vivo evaluation. Mol. Pharm. 2011, 8, 1209–1223.
- Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable vaginal hydrogels as a multi-drug carrier for contraception. Appl. Sci. 2019, 9, 1638.
- Bahamondes, L.; Bahamondes, M.V. New and emerging contraceptives: A state-of-the-art review. Int. J. Womens Health 2014, 6, 221.
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509.
- das Neves, J.; Nunes, R.; Rodrigues, F.; Sarmento, B. Nanomedicine in the development of anti-HIV microbicides. Adv. Drug Deliv. Rev. 2016, 103, 57–75.
- Lakshmi, Y.S.; Kumar, P.; Kishore, G.; Bhaskar, C.; Kondapi, A.K. Triple combination MPT vaginal microbicide using curcumin and efavirenz loaded lactoferrin nanoparticles. Sci. Rep. 2016, 6, 25479.
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; das Neves, J. Pharmaceutical vehicles for vaginal and rectal administration of anti-HIV microbicide nanosystems. Pharmaceutics 2019, 11, 145.
- Li, Z.; Cho, S.; Kwon, I.C.; Janát-Amsbury, M.M.; Huh, K.M. Preparation and characterization of glycol chitin as a new thermogelling polymer for biomedical applications. Carbohydr. Polym. 2013, 92, 2267–2275.
- Almomen, A.; Cho, S.; Yang, C.-H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279.
- Arun Karthick, R.; Ramya Devi, D.; Vedha Hari, B.N. Investigation of sustained release mucoadhesive in-situ gel system of Secnidazole for the persistent treatment of vaginal infections. J. Drug Deliv. Sci. Technol. 2018, 43, 362–368.
- Patel, P.; Patel, P. Formulation and evaluation of clindamycin HCL in situ gel for vaginal application. Int. J. Pharm. Investig. 2015, 5, 50.
- Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Mendes, E.; Dubruel, P. Cross-linkable, thermo-responsive Pluronic® building blocks for biomedical applications: Synthesis and physico-chemical evaluation. Eur. Polym. J. 2014, 53, 126–138.
- Lu, C.; Liu, M.; Fu, H.; Zhang, W.; Peng, G.; Zhang, Y.; Cao, H.; Luo, L. Novel thermosensitive in situ gel based on poloxamer for uterus delivery. Eur. J. Pharm. Sci. 2015, 77, 24–28.
- Soliman, G.M.; Fetih, G.; Abbas, A.M. Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: In vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation. Drug Dev. Ind. Pharm. 2017, 43, 399–408.
- Abd Ellah, N.H.; Abouelmagd, S.A.; Abbas, A.M.; Shaaban, O.M.; Hassanein, K.M.A. Dual-responsive lidocaine in situ gel reduces pain of intrauterine device insertion. Int. J. Pharm. 2018, 538, 279–286.
- Yang, T.-T.; Cheng, Y.-Z.; Qin, M.; Wang, Y.-H.; Yu, H.-L.; Wang, A.-L.; Zhang, W.-F. Thermosensitive chitosan hydrogels containing polymeric microspheres for vaginal drug delivery. Biomed. Res. Int. 2017, 2017, 3564060.
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological behaviour of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2011, 83, 1128–1133.
- Dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867.
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138.
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54.
- Pandey, P.; Cabot, P.J.; Wallwork, B.; Panizza, B.J.; Parekh, H.S. Formulation, functional evaluation and ex vivo performance of thermoresponsive soluble gels-A platform for therapeutic delivery to mucosal sinus tissue. Eur. J. Pharm. Sci. 2017, 96, 499–507.
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671.
- Ci, L.; Huang, Z.; Liu, Y.; Liu, Z.; Wei, G.; Lu, W. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: Preparation, characterization and application in a vaginal drug delivery system. Acta Pharm. Sin. B 2017, 7, 593–602.
- Barros, S.C.; da Silva, A.A.; Costa, D.B.; Cesarino, I.; Costa, C.M.; Lanceros-Méndez, S.; Pawlicka, A.; Silva, M.M. Thermo-sensitive chitosan–cellulose derivative hydrogels: Swelling behaviour and morphologic studies. Cellulose 2014, 21, 4531–4544.
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349.
- Chatterjee, S.; Hui, P.C.-L. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086.
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6.
- Chen, D.; Yu, H.; Sun, K.; Liu, W.; Wang, H. Dual thermoresponsive and pH-responsive self-assembled micellar nanogel for anticancer drug delivery. Drug Deliv. 2014, 21, 258–264.
- Qin, C.; Zhou, J.; Zhang, Z.; Chen, W.; Hu, Q.; Wang, Y. Convenient one-step approach based on stimuli-responsive sol-gel transition properties to directly build chitosan-alginate core-shell beads. Food Hydrocoll. 2019, 87, 253–259.
- Cirri, M.; Maestrelli, F.; Scuota, S.; Bazzucchi, V.; Mura, P. Development and microbiological evaluation of chitosan and chitosan-alginate microspheres for vaginal administration of metronidazole. Int. J. Pharm. 2021, 598, 120375.
- Moreno, M.A.; Gómez-Mascaraque, L.G.; Arias, M.; Zampini, I.C.; Sayago, J.E.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; López-Rubio, A.; Isla, M.I. Electrosprayed chitosan microcapsules as delivery vehicles for vaginal phytoformulations. Carbohydr. Polym. 2018, 201, 425–437.
- Rodriguez-Tenreiro, C.; Diez-Bueno, L.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Cyclodextrin/carbopol micro-scale interpenetrating networks (ms-IPNs) for drug delivery. J. Control. Release 2007, 123, 56–66.
- Migliozzi, S.; Angeli, P.; Mazzei, L. Gelation kinetics of non-aqueous Carbopol dispersions. Colloids Surf. B 2019, 577, 84–95.
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158.
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016.
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152.
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389.
- Mishra, R.; Soni, K.; Mehta, T. Mucoadhesive vaginal film of fluconazole using cross-linked chitosan and pectin. J. Therm. Anal. Calorim. 2017, 130, 1683–1695.
- Bakke, A.J.; Zaveri, T.; Higgins, M.J.; Ziegler, G.R.; Hayes, J.E. Design aspects of vaginal applicators that influence acceptance among target users. Sci. Rep. 2021, 11, 9802.
- Montesino, M.; Labrie, F.; Archer, D.F.; Zerhouni, J.; Côté, I.; Lavoie, L.; Beauregard, A.; Martel, C.; Vaillancourt, M.; Moyneur, E. Evaluation of the acceptability of intravaginal prasterone ovule administration using an applicator. Gynecol. Endocrinol. 2016, 32, 240–245.
- Das Neves, J.; Notario-Pérez, F.; Sarmento, B. Women-specific routes of administration for drugs: A critical overview. Adv. Drug Deliv. Rev. 2021, 176, 113865.
- Omar, R.F.; Trottier, S.; Brousseau, G.; Ouellet, C.; Danylo, A.; Ong, T.; Bergeron, M.G. Universal vaginal applicator for the uniform distribution of vaginal gel and cream formulations: A magnetic resonance imaging study. J. Obstet. Gynaecol. Can. 2014, 36, 42–50.
- Coppola, J.S. A New Vaginal pH Regulator for Hormone-Free, On-Demand Contraception. Nurs. Womens Health 2021, 25, 152–155.
- Brache, V.; Cohen, J.A.; Cochon, L.; Alvarez, F. Evaluating the clinical safety of three vaginal applicators: A pilot study conducted in the Dominican Republic. Contraception 2006, 73, 72–77.
- Brunner, H.; Theodor, R.A. Multiple use applicator for vaginal tablets/vaginal inserts: Compliance verification and suitability studies. BMC Womens Health 2020, 20, 235.
