Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 3 by Lindsay Dong.
Not all venoms contain the same constituents; not all sensory neurons or other components of the nervous system are vulnerable to the same peptide or enzyme; not all tissues and organs have the same innervation or vulnerability to venom constituents; and, lastly, snakes have incredibly diverse venom proteomes, a diversity driven by geographical and other environmental factors. Documentation of specific pain syndromes in greater detail in future epidemiological studies of snake bite is also critical.
- neurotoxicity
- venomous snake bite
- acute pain
- chronic pain
- phospholipase A2
- serine protease
- metalloproteinase
- snake venom peptides
1. Introduction
A primordial fear shared by humankind is often heralded by the sudden onset of intense pain in a limb—the beginning of envenomation and neurotoxicity from a snake bite. This is only the onset of what may be a complex experience composed of fear, injury to limb and potentially loss of life. However, in the maelstrom of this event, the cause and nature of the painful experience associated with envenomation may be as varied as the biochemical and proteomic composition of the venom [1][2][3] to which the bitten are exposed. Acute local pain [4][5] can spread systemically, experienced as headache [6][7][8][9][10][11][12][13][14][15][16], eye pain [17][18][19][20][21][22][23][24][25][26][27][28], chest pain [27][28][29][30][31][32][33][34], focal back pain [34][35], abdominal pain [5][33][36][37][38][39][40][41][42][43][44][45][46] or generalized pain [34][38][39] that may last only during the immediate episode or can progress into chronic pain syndromes such as migraine headache or complex regional pain syndrome (CRPS) [7][47][48][49][50][51][52]. Given the complex and seemingly unpredictable outcome in the matter of snake venom induced pain, it would be important to understand the molecular mechanisms underlying this experience that subsequently dictate appropriate treatments.
2. Location: Molecular Mechanisms of Venom Mediated Pain
2.1. Overview
The first paradigm of location concerns the molecular site of action that causes pain. The molecular composition of venom obtained from snakes can be remarkably complex, composed of biogenic amines, enzymes, peptides, and other substances to incapacitate their prey [1][2][3][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72]. The reader is referred to a few recent excellent reviews for greater detail [57][58][59][62]. Examples of such compounds include proteins, small molecular weight, non-enzymatic compounds, serine proteases, metalloproteinases, phospholipase A2, and 3-finger toxins [1][2][3][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72]. These venom components or their enzymatic byproducts interact with a variety of receptors on Aδ and C pain fibers as recently reviewed [58][59][60]. The following subsections outline the likely mechanisms by which these venom components inflict pain in a myriad of ways.
The paradigm invoked by the aforementioned is that the venom proteins presented do in fact inflict pain either directly by stimulating specific receptors by binding to the receptors or locally generating compounds that bind, remotely by stimulating receptors distant from the bite site, or indirectly by causing pain syndromes as a result of ischemia or relentless muscle activation. While the administration of antivenom does attenuate pain syndromes in areas remote from the bite site by binding to and neutralizing circulating and locally presented venom compounds, tissue edema and relative tissue ischemia surrounding the bite site may prevent attenuation of pain secondary to antivenom not being able to be delivered to the envenomation strike point. Individual classifications of venom compound and molecular site of action are subsequently presented.
2.2. Small Molecular Weight, Non-Enzymatic Compounds (Direct Effects)
This category includes compounds such as biogenic amines (e.g., histamine, serotonin), kinins, eicosanoids, and other peptides that bind to their specific receptors [53][54][55][56][57][58][59][60]. These are found as preformed substances in venoms to varying degrees [53][54][55][56][57][58][59][60] and likely contribute to the initial pain sensations after being bitten. Most of these substances are rapidly metabolized in the envenomed tissues, circulation, or lung by enzymes such as histaminase, monoamine oxidase, 15-hydroxyprostaglandin dehydrogenase, and cytochrome 450s. Thus, without ongoing generation, the aforementioned preformed small molecular weight compounds would be expected to contribute to pain experienced at the bite site but not at distant sites, and only for a brief time. An exception could be non-enzymatic proteins, such as those in the venom of Echis coloratus, that activate the transient receptor potential vanilloid 1 (TRPV1) channel [60], which could leave the bite site without being degraded to cause pain elsewhere in the victim. Nevertheless, and critically, the vast majority of venomous snake bites are not as severe initially as they are in the minutes or hours that follow envenomation [4]. When considered as a whole, it is likely that the formation of these and other compounds via catalysis of the envenomed tissues by enzymes contained in venom contributes to progressively increasing local pain. However, as the subsequently described venom proteins are released into the circulation and cause pain in distant organs in a syndromic fashion, it should be remembered that as end-organ inflammation increases, so does release of the aforementioned small molecular weight compounds that may contribute to pain systemically. In summary, while preformed substances in venom likely cause early pain, it is the subsequently described enzyme classes contained in venom that contribute to pain at the bite site and in locations distant from the initial strike point.
2.3. Phospholipase A2 (PLA2) (Direct Effects)
While these snake venom enzymes are perhaps most feared for their properties as preganglionic neurotoxins (β-neurotoxins) that inflict apneic death [1][57][58][59], they also cause edema, tissue injury and, critically, pain [57][58][59][62][72][73][74][75][76]. PLA2 activity catalyzes phospholipids in the bite site and beyond after release into the circulation, resulting in formation of bradykinin, biogenic amines, prostaglandins, and other compounds that inflict pain [57][58][62][72][73][74][75][76]. For example, PLA2 isolated from Crotalus durissus species venom has been demonstrated to activate C fibers, resulting in the release of substance P, mast cell degranulation, and finally, release of histamine and serotonin [63]. A similar release of substance P and bradykinin was observed following the use of a secretory PLA2 isolated from Naja mocambique mocambique venom in a model of acute pancreatitis [64]. In the same vein, a PLA2 isolated from Micruruus lemniscatus, Lemnitoxin, was found to be a potent agent that degranulated mast cells [65]. A PLA2 isolated from Bothrops atrox venom, BatroxPLA2, caused release of IL-6 and formation of prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and cysteinyl leukotrienes (CysLTs) in mice [66]. Further, snake venom PLA2 from Bothrops species also inflict pain via cellular release of adenosine triphosphate and potassium [75][76]. Of interest, a heteromeric toxin composed of a PLA2 with minimal enzymatic activity with Kunitz-like protein (MitTx) purified from the venom of Micrurus tener tener that activates acid-sensing ion channels (ASICs) independent of enzymatic activity has been identified as a source of pain [61]. Of equal importance, the role of PLA2 in the development of pain has been demonstrated by inhibition of these enzymes, which results in a decrease in pain in vivo [77][78]. For the interested reader, a more in-depth consideration of PLA2 is recommended [62]. Thus, it is likely that PLA2 significantly contribute to the pain syndromes subsequently presented.
2.4. Serine Proteases (Direct and Indirect Effects)
This class of snake venom enzyme is perhaps most notorious for inflicting coagulopathy following snake bite [1]; however, these enzymes are also demonstrated to contribute to pain in more than one manner. Serine proteases activate protease-activated receptor 2 (PAR2), which in turn generates pain in several settings [79][80]. Using murine models, human cancer cells secrete serine proteases that inflict pain when injected into the hind paw, and this pain was reduced with serine protease inhibitors [79]. In another investigation, the pain caused by injection of mice paws with formalin, bradykinin, or PAR2-activating peptide was reduced in animals with PAR2 deletion [79]. As for an example with snake venom, serine proteases purified from the venom of Bothrops pirajai significantly contributed to hyperalgesia in a murine paw bending model [67]. A second mechanism by which serine proteases may inflict pain is by causing regional arterial thrombosis via activation of coagulation [1], which would result in regional ischemic pain. Examples of ischemic pain will be presented in detail in the following sections. In summary, serine proteases likely play a significant role in envenomation associated pain.
2.5. Metalloproteinases (Direct and Indirect Effects)
Metalloproteinases also have a variety of proven or possible mechanisms by which they may contribute to snake bite pain, and there are several examples found in the literature. A metalloproteinase purified from Bothrops atrox, Batroxase, caused release of IL-6 and formation of PGE2, LTB4, and CysLTs in mice [66][71]. Further, metalloproteinases contained in Bothrops jararaca venom enhanced hyperalgesia in a murine model [68], as did a purified metalloproteinase, BaP1, contained in Bothrops asper venom, via TNF-α and PGE2-dependent mechanisms [69]. A final example is the hyperalgesic effect of a metalloproteinase, BpirMP, in a rat model that was purified from the venom of Bothrops pirajai [70]. As for other mechanisms, these enzymes have been associated with neuropathic pain, with cleavage of interleukin-1β resulting in the activation of microglial cells or astrocytes, depending on the metalloproteinase involved [81]. Further, similar to serine proteases, metalloproteinases are capable of activating PAR2 [82]. Lastly, this class of enzyme can exert potent procoagulant activity, resulting in arterial thrombosis and ischemic pain [1].
2.6. Fasciculins (Indirect Effects)
Fasciculins, found in Dendroaspis species (mambas) venom, are a subclass of three-finger toxins that exert their toxicity by causing uncontrollable fasciculations of skeletal muscle and subsequent paralysis and apneic death [2]. In addition to paralysis, fasciculations are painful, and continuous fasciculation can result in significant muscle damage and pain after recovering from the snake bite despite mechanical ventilation and pharmacological neuromuscular blockade [83]. Fasciculins bind to circulating acetylcholinesterase and inactivate the enzyme, allowing continuous exposure of the post synaptic membrane of neuromuscular junctions to acetylcholinesterase, resulting in fasciculations [84]. Similar pain, but to a far lesser degree, is observed postoperatively in muscular patients after administration of succinylcholine during the conduct of anesthetic induction [85]. This medication briefly (1–2 min) depolarizes skeletal muscle to effect temporary paralysis to facilitate endotracheal intubation, and the musculature is observed briefly to fasciculate [86]. Therefore, it is not surprising that patients that survive a mamba bite may complain of significant muscular pain afterwards [83]. Thus, fasciculins are a unique indirectly acting, pain-provoking agent in snake venom.
A diagrammatic and simplified summary of this section is provided in Figure 1. For a detailed review of the cellular and molecular mechanisms of pain, the interested reader is referred to an excellent review [86].
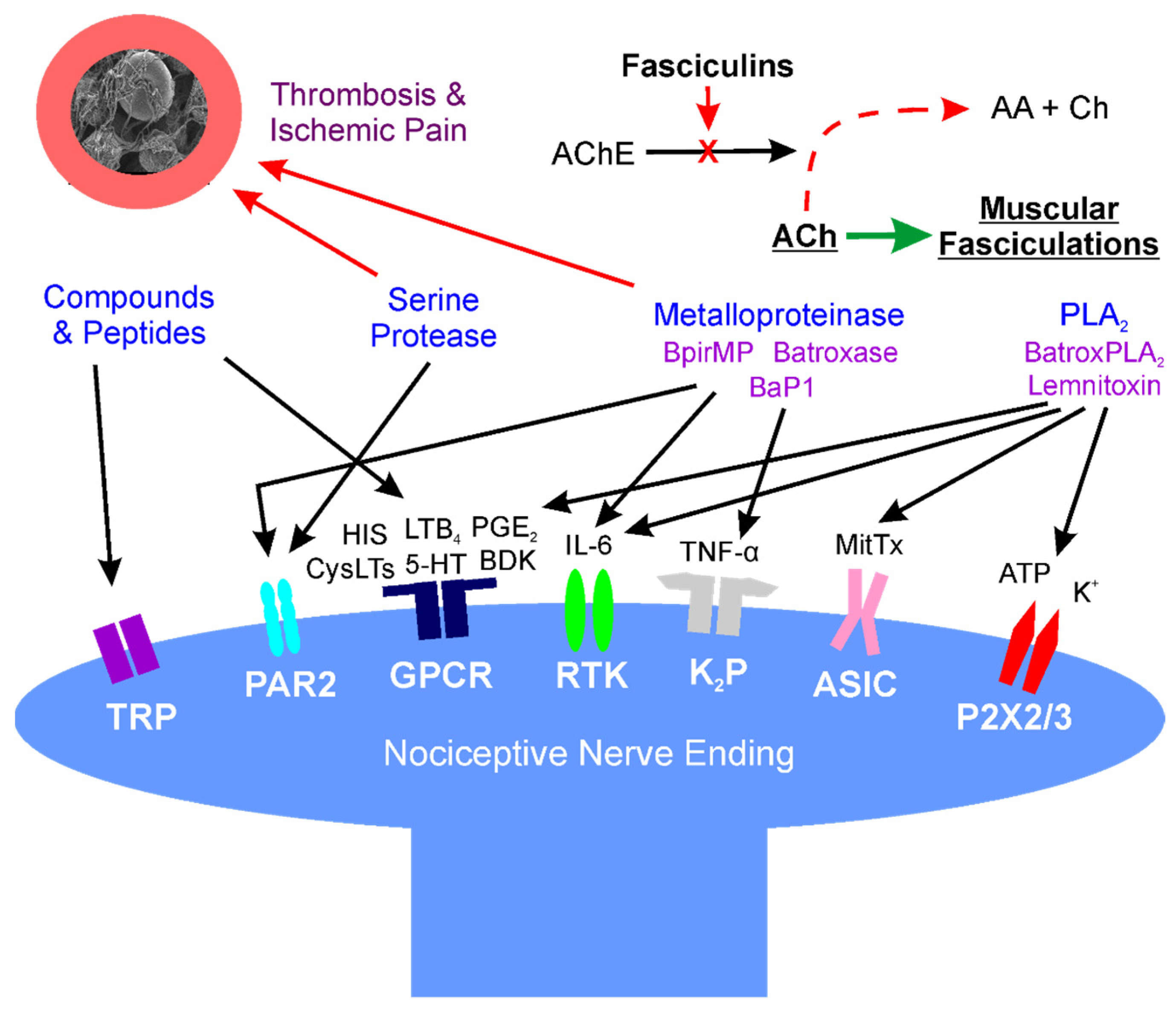 Figure 1. Location: molecular mechanisms of venom mediated pain. Diagram of interactions of snake venom compounds and proteins with nociceptive nerve endings and other key systems that result in pain. As explained in detail in the text, the indicated compounds and proteins activate receptors either directly or via products of enzymatic catalysis. Further, arterial thrombosis and ischemic pain remote from the bite are caused by serine proteases and metalloproteinases; also, at neuromuscular junctions distant from the bite, fasciculins inactivate acetylcholinesterase activity, allowing relentless activation of muscular activity via acetylcholine. AA—acetic acid; AChE—acetylcholinesterase; ASIC—acid sensing ion channel; ATP—adenosine triphosphate; BaP1, Batroxase, BpirMP—examples of metalloproteinases; BatroxPLA2, Lemnitoxin—examples of PLA2; BDK—bradykinin; Ch—choline; CysLTs, LTB4—examples of leukotrienes; GPCR—G-protein coupled receptor; HIS—histamine; IL-6—interleukin 6; K+—potassium; K2P—two-pore potassium channel; MitTx—a low activity PLA2 molecule bound to a with Kunitz-like protein that directly activates ASIC; P2X2/3—purinoceptors 2X2 and 2X3; PAR2—protease-activated receptor 2; PGE2—prostaglandin E2; RTK—receptor tyrosine kinase; and, TNF-α—tumor necrosis factor-α; TRP—transient receptor potential channel.
Figure 1. Location: molecular mechanisms of venom mediated pain. Diagram of interactions of snake venom compounds and proteins with nociceptive nerve endings and other key systems that result in pain. As explained in detail in the text, the indicated compounds and proteins activate receptors either directly or via products of enzymatic catalysis. Further, arterial thrombosis and ischemic pain remote from the bite are caused by serine proteases and metalloproteinases; also, at neuromuscular junctions distant from the bite, fasciculins inactivate acetylcholinesterase activity, allowing relentless activation of muscular activity via acetylcholine. AA—acetic acid; AChE—acetylcholinesterase; ASIC—acid sensing ion channel; ATP—adenosine triphosphate; BaP1, Batroxase, BpirMP—examples of metalloproteinases; BatroxPLA2, Lemnitoxin—examples of PLA2; BDK—bradykinin; Ch—choline; CysLTs, LTB4—examples of leukotrienes; GPCR—G-protein coupled receptor; HIS—histamine; IL-6—interleukin 6; K+—potassium; K2P—two-pore potassium channel; MitTx—a low activity PLA2 molecule bound to a with Kunitz-like protein that directly activates ASIC; P2X2/3—purinoceptors 2X2 and 2X3; PAR2—protease-activated receptor 2; PGE2—prostaglandin E2; RTK—receptor tyrosine kinase; and, TNF-α—tumor necrosis factor-α; TRP—transient receptor potential channel.
 Figure 1. Location: molecular mechanisms of venom mediated pain. Diagram of interactions of snake venom compounds and proteins with nociceptive nerve endings and other key systems that result in pain. As explained in detail in the text, the indicated compounds and proteins activate receptors either directly or via products of enzymatic catalysis. Further, arterial thrombosis and ischemic pain remote from the bite are caused by serine proteases and metalloproteinases; also, at neuromuscular junctions distant from the bite, fasciculins inactivate acetylcholinesterase activity, allowing relentless activation of muscular activity via acetylcholine. AA—acetic acid; AChE—acetylcholinesterase; ASIC—acid sensing ion channel; ATP—adenosine triphosphate; BaP1, Batroxase, BpirMP—examples of metalloproteinases; BatroxPLA2, Lemnitoxin—examples of PLA2; BDK—bradykinin; Ch—choline; CysLTs, LTB4—examples of leukotrienes; GPCR—G-protein coupled receptor; HIS—histamine; IL-6—interleukin 6; K+—potassium; K2P—two-pore potassium channel; MitTx—a low activity PLA2 molecule bound to a with Kunitz-like protein that directly activates ASIC; P2X2/3—purinoceptors 2X2 and 2X3; PAR2—protease-activated receptor 2; PGE2—prostaglandin E2; RTK—receptor tyrosine kinase; and, TNF-α—tumor necrosis factor-α; TRP—transient receptor potential channel.
Figure 1. Location: molecular mechanisms of venom mediated pain. Diagram of interactions of snake venom compounds and proteins with nociceptive nerve endings and other key systems that result in pain. As explained in detail in the text, the indicated compounds and proteins activate receptors either directly or via products of enzymatic catalysis. Further, arterial thrombosis and ischemic pain remote from the bite are caused by serine proteases and metalloproteinases; also, at neuromuscular junctions distant from the bite, fasciculins inactivate acetylcholinesterase activity, allowing relentless activation of muscular activity via acetylcholine. AA—acetic acid; AChE—acetylcholinesterase; ASIC—acid sensing ion channel; ATP—adenosine triphosphate; BaP1, Batroxase, BpirMP—examples of metalloproteinases; BatroxPLA2, Lemnitoxin—examples of PLA2; BDK—bradykinin; Ch—choline; CysLTs, LTB4—examples of leukotrienes; GPCR—G-protein coupled receptor; HIS—histamine; IL-6—interleukin 6; K+—potassium; K2P—two-pore potassium channel; MitTx—a low activity PLA2 molecule bound to a with Kunitz-like protein that directly activates ASIC; P2X2/3—purinoceptors 2X2 and 2X3; PAR2—protease-activated receptor 2; PGE2—prostaglandin E2; RTK—receptor tyrosine kinase; and, TNF-α—tumor necrosis factor-α; TRP—transient receptor potential channel.References
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576.
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123.
- Villar-Briones, A.; Aird, S.D. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins 2018, 10, 392.
- Ward-Smith, H.; Arbuckle, K.; Naude, A.; Wüster, W. Fangs for the Memories? A Survey of Pain in Snakebite Patients Does Not Support a Strong Role for Defense in the Evolution of Snake Venom Composition. Toxins 2020, 12, 201.
- Chippaux, J.P.; Amri, K. Severe Heloderma spp. envenomation: A review of the literature. Clin. Toxicol. 2021, 59, 179–184.
- Tibballs, J. Diagnosis and treatment of confirmed and suspected snake bite. Implications from an analysis of 46 paediatric cases. Med. J. Aust. 1992, 156, 270–274.
- Jayawardana, S.; Arambepola, C.; Chang, T.; Gnanathasan, A. Long-term health complications following snake envenoming. J. Multidiscip. Healthc. 2018, 11, 279–285.
- Alves, E.C.; Sachett, J.A.G.; Sampaio, V.S.; Sousa, J.D.B.; Oliveira, S.S.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, e0202361.
- Razavi, S.; Weinstein, S.A.; Bates, D.J.; Alfred, S.; White, J. The Australian mulga snake (Pseudechis australis: Elapidae): Report of a large case series of bites and review of current knowledge. Toxicon 2014, 85, 17–26.
- Tilbury, C.R.; Branch, W.R. Observations on the bite of the southern burrowing asp (Atractaspis bibronii) in Natal. S. Afr. Med. J. 1989, 75, 327–331.
- Jansen, M.; McLeod, M.; White, J.; Isbister, G.K. Spotted black snake (Pseudechis guttatus) envenoming. Med. J. Aust. 2007, 186, 41–42.
- Medeiros, C.R.; Souza, S.N.; Silva, M.C.D.; Ventura, J.S.; Piorelli, R.O.; Puorto, G. Bites by Tomodon dorsatus (serpentes, dipsadidae): Clinical and epidemiological study of 86 cases. Toxicon 2019, 162, 40–45.
- Gan, M.; O’Leary, M.A.; Brown, S.G.; Jacoby, T.; Spain, D.; Tankel, A.; Gavaghan, C.; Garrett, P.; Isbister, G.K. Envenoming by the rough-scaled snake (Tropidechis carinatus): A series of confirmed cases. Med. J. Aust. 2009, 191, 183–186.
- Karlo, R.; Dželalija, B.; Zupančić, B.; Bačić, I.; Dunatov, T.; Kanjer, A.; Skarica, R.; Sabalić, S.; Bukvic, N.; Nikolić, H.; et al. Venomous snakebites in the Croatian North Dalmatia region. Wien. Klin. Wochenschr. 2011, 123, 732–737.
- Nascimento da Costa, T.; Mota-da-Silva, A.; Colombini, M.; Moura-da-Silva, A.M.; Medeiros de Souza, R.; Monteiro, W.M.; Bernarde, P.S. Relationship between snake size and clinical, epidemiological and laboratory aspects of Bothrops atrox snakebites in the Western Brazilian Amazon. Toxicon 2020, 186, 160–167.
- Hansen, E.A.; Stein, E.A.; Mader, T.H.; Mazzoli, R.A. Spitting cobra ophthalmia in United Nations Forces in Somalia. Am. J. Ophthalmol. 1994, 117, 671.
- Chu, E.R.; Weinstein, S.A.; White, J.; Warrell, D.A. Venom ophthalmia caused by venoms of spitting elapid and other snakes: Report of ten cases with review of epidemiology, clinical features, pathophysiology and management. Toxicon 2010, 56, 259–272.
- Ang, L.J.; Sanjay, S.; Sangtam, T. Ophthalmia due to spitting cobra venom in an urban setting--a report of three cases. Middle East Afr. J. Ophthalmol. 2014, 21, 259–261.
- Lanzetta, M.A.; Cirtita, M.; Aziebu, E.; Cham, M.; Lanzetta, P. Ophthalmia Secondary to Cobra Venom Spitting in the Volta Region, Ghana: A Case Report. Case Rep. Ophthalmol. 2017, 8, 99–103.
- Tsai, T.H.; Lin, C.C.; Mao, Y.C.; Hung, C.L.; Yang, Y.C.; Yang, C.C.; Jeng, M.J. Naja atra venom-spit ophthalmia in Taiwan: An epidemiological survey from 1990 to 2016. J. Chin. Med. Assoc. 2020, 83, 77–83.
- Sai-Sein-Lin-Oo; Myat-Thet-New; Khin-Maung-Gyi; Than-Aye; Mi-Mi-Khine; Myat-Myat-Thein; Myo-Thant; Pyae-Phyo-Aung; Oakkar-Kyaw-Khant; Aye-Zarchi-San; et al. Clinical importance of the Mandalay spitting cobra (Naja mandalayensis) in Upper Myanmar-Bites, envenoming and ophthalmia. Toxicon 2020, 184, 39–47.
- Chang, K.C.; Huang, Y.K.; Chen, Y.W.; Chen, M.H.; Tu, A.T.; Chen, Y.C. Venom Ophthalmia and Ocular Complications Caused by Snake Venom. Toxins 2020, 12, 576.
- Handford, C. Case of venom ophthalmia following contact with Naja pallida: The red spitting cobra. J. R. Army Med. Corps. 2018, 164, 124–126.
- Johnson, R. Ophthalmic Exposure to Crotalid Venom. J. Emerg. Med. 2009, 36, 37–38.
- Chen, Y.C.; Yen, D.H.; Chen, Y.W.; Huang, M.S.; Huang, C.I.; Chen, M.H. Toxin ophthalmia caused by nuchal gland secretion of the Taiwan tiger keelback (Rhabdophis tigrinus formosanus). J. Formos. Med. Assoc. 2014, 113, 750–753.
- Chu, E.R.; White, J.; Weinstein, S. Is there any role for intravenous antivenom for snake venom ophthalmia? J. Emerg. Med. 2010, 39, 659–660.
- Dissanayake, P.; Sellahewa, K.H. Acute myocardial infarction in a patient with Russell’s viper bite. Ceylon Med. J. 1996, 41, 67–68.
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science 2021, 371, 386–390.
- Simpson, C.H.; Richardson, W.H.; Swartzentruber, G.S.; Lloyd, V.J. ST Segment Elevation Myocardial Infarction Following a Crotalus horridus Envenomation. Wilderness Environ. Med. 2018, 29, 383–387.
- Frangides, C.; Kouni, S.; Niarchos, C.; Koutsojannis, C. Hypersersensitivity and Kounis syndrome due to a viper bite. Eur. J. Intern. Med. 2006, 17, 215–216.
- Saadeh, A.M. Case report: Acute myocardial infarction complicating a viper bite. Am. J. Trop. Med. Hyg. 2001, 64, 280–282.
- Satish, R.; Kanchan, R.; Yashawant, R.; Ashish, D.; Kedar, R. Acute MI in a stented patient following snake bite-possibility of stent thrombosis-A case report. Indian Heart J. 2013, 65, 327–330.
- Bawaskar, H.S.; Bawaskar, P.H.; Bawaskar, P.H. Premonitory signs and symptoms of envenoming by common krait (Bungarus caeruleus). Trop. Doct. 2014, 44, 82–85.
- Valenta, J.; Stach, Z.; Fricova, D.; Zak, J.; Balik, M. Envenoming by the viperid snake Proatheris superciliaris: A case report. Toxicon 2008, 52, 392–394.
- Pourreau, F.; Pinsard, M.; Goyffon, M.; Plasse, F.; Desport, E.; Thierry, A.; Touchard, G.; Bridoux, F. Bilateral renal cortical necrosis with end-stage renal failure following envenoming by Proatheris superciliaris: A case report. Toxicon 2014, 84, 36–40.
- Sarkhel, S.; Ghosh, R.; Mana, K.; Gantait, K. A hospital based epidemiological study of snakebite in Paschim Medinipur district, West Bengal, India. Toxicol. Rep. 2017, 4, 415–419.
- Atkinson, P.M.; Bradlow, B.A.; White, J.A.; Greig, H.B.; Gaillard, M.C. Clinical features of twig snake (Thelotornis capensis) envenomation. S. Afr. Med. J. 1980, 58, 1007–1011.
- Kularatne, S.A. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: A prospective clinical study, 1996–1998. Postgrad. Med. J. 2002, 78, 276–280.
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Liao, S.C.; Su, H.Y.; Hsieh, S.Y.; Yang, C.C. Bungarus multicinctus multicinctus Snakebite in Taiwan. Am. J. Trop. Med. Hyg. 2017, 96, 1497–1504.
- Bucaretchi, F.; Herrera, S.R.; Hyslop, S.; Baracat, E.C.; Vieira, R.J. Snakebites by Bothrops spp. in children in Campinas, São Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 329–333.
- Singh, J.; Bhoi, S.; Gupta, V.; Goel, A. Clinical profile of venomous snake bites in north Indian Military Hospital. J. Emerg. Trauma Shock 2008, 1, 78–80.
- Pivko-Levy, D.; Munchnak, I.; Rimon, A.; Balla, U.; Scolnik, D.; Hoyte, C.; Voliovitch, Y.; Glatstein, M. Evaluation of antivenom therapy for Vipera palaestinae bites in children: Experience of two large, tertiary care pediatric hospitals. Clin. Toxicol. 2017, 55, 235–240.
- Kularatne, S.A.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, e90198.
- Hermansen, M.N.; Krug, A.H.; Tjønnfjord, E.; Brabrand, M. Envenomation by the common European adder (Vipera berus): A case series of 219 patients. Eur. J. Emerg. Med. 2019, 26, 362–365.
- Paolino, G.; Di Nicola, M.R.; Pontara, A.; Didona, D.; Moliterni, E.; Mercuri, S.R.; Grano, M.; Borgianni, N.; Kumar, R.; Pampena, R. Vipera snakebite in Europe: A systematic review of a neglected disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2247–2260.
- Reid, H.A. Adder bites in Britain. Br. Med. J. 1976, 2, 153–156.
- Bhattarai, B.; Shrestha, B.P.; Rahman, T.R.; Sharma, S.K.; Tripathi, M. Complex regional pain syndrome (CRPS) type-1 following snake bite: A case report. Nepal Med. Coll. J. 2008, 10, 278–280.
- Seo, Y.H.; Park, M.R.; Yoo, S.H. Development of complex regional pain syndrome after a snake bite: A case report. Korean J. Pain. 2014, 27, 68–71.
- Pachowicz, M.; Nocuń, A.; Postępski, J.; Olesińska, E.; Emeryk, A.; Chrapko, B. Complex Regional Pain Syndrome type I with atypical scintigraphic pattern–diagnosis and evaluation of the entity with three phase bone scintigraphy. A case report. Nucl. Med. Rev. Cent. East. Eur. 2014, 17, 115–119.
- Kleggetveit, I.P.; Skulberg, P.K.; Jørum, E. Complex regional pain syndrome following viper-bite. Scand. J. Pain. 2016, 10, 15–18.
- Cruz Salcedo, E.M.; Blanco, A.; Reed, J. Complex Regional Pain Syndrome Developing After a Coral Snake Bite: A Case Report. Cureus 2020, 12, e9787.
- Lazaro, R.P. Complex Regional Pain Syndrome Following Snakebite: A Putatively Rare Complication of Envenomation and Review of the Literature. Int. Med. Case Rep. J. 2020, 13, 603–607.
- Welsh, J.H. Serotonin and related tryptamine derivatives in snake venoms. Mem. Inst. Butantan. 1966, 33, 509–518.
- Lopes, P.H.; Rocha, M.M.T.; Kuniyoshi, A.K.; Portaro, F.C.V.; Gonçalves, L.R.C. Edema and Nociception Induced by Philodryas patagoniensis Venom in Mice: A Pharmacological Evaluation with Implications for the Accident Treatment. J. Pharm. Exp. Ther. 2017, 361, 349–354.
- Maia-Marques, R.; Nascimento, I.M.R.; Lauria, P.S.S.; Silva, E.C.P.D.; Silva, D.F.; Casais-E-Silva, L.L. Inflammatory mediators in the pronociceptive effects induced by Bothrops leucurus snake venom: The role of biogenic amines, nitric oxide, and eicosanoids. Toxicology 2021, 448, 152649.
- Freedman, J.E.; Snyder, S.H. Vipoxin. A protein from Russell’s viper venom with high affinity for biogenic amine receptors. J. Biol. Chem. 1981, 256, 13172–13179.
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218.
- Jami, S.; Erickson, A.; Brierley, S.M.; Vetter, I. Pain-Causing Venom Peptides: Insights into Sensory Neuron Pharmacology. Toxins 2017, 10, 15.
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers. 2017, 3, 17079.
- Geron, M.; Kumar, R.; Matzner, H.; Lahiani, A.; Gincberg, G.; Cohen, G.; Lazarovici, P.; Priel, A. Protein toxins of the Echis coloratus viper venom directly activate TRPV1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 615–623.
- Bohlen, C.J.; Chesler, A.T.; Sharif-Naeini, R.; Medzihradszky, K.F.; Zhou, S.; King, D.; Sánchez, E.E.; Burlingame, A.L.; Basbaum, A.I.; Julius, D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011, 479, 410–414.
- Costa, S.K.P.; Camargo, E.A.; Antunes, E. Inflammatory Action of Secretory Phospholipases A2 from Snake Venoms. In Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L., Luo, S., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 35–52.
- Câmara, P.R.; Esquisatto, L.C.; Camargo, E.A.; Ribela, M.T.; Toyama, M.H.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by phospholipases A2 isolated from Crotalus durissus sp. in the rat dorsal skin: A role for mast cells and sensory C-fibers. Toxicon 2003, 41, 823–829.
- Camargo, E.A.; Ferreira, T.; Ribela, M.T.; de Nucci, G.; Landucci, E.C.; Antunes, E. Role of substance P and bradykinin in acute pancreatitis induced by secretory phospholipase A2. Pancreas 2008, 37, 50–55.
- Casais-E-Silva, L.L.; Teixeira, C.F.; Lebrun, I.; Lomonte, B.; Alape-Girón, A.; Gutiérrez, J.M. Lemnitoxin, the major component of Micrurus lemniscatus coral snake venom, is a myotoxic and pro-inflammatory phospholipase A2. Toxicol. Lett. 2016, 257, 60–71.
- Menaldo, D.L.; Bernardes, C.P.; Zoccal, K.F.; Jacob-Ferreira, A.L.; Costa, T.R.; Del Lama, M.P.; Naal, R.M.; Frantz, F.G.; Faccioli, L.H.; Sampaio, S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 2017, 85, 238–247.
- Menaldo, D.L.; Bernardes, C.P.; Pereira, J.C.; Silveira, D.S.; Mamede, C.C.; Stanziola, L.; de Oliveira, F.; Pereira-Crott, L.S.; Faccioli, L.H.; Sampaio, S.V. Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int. Immunopharmacol. 2013, 15, 764–771.
- Zychar, B.C.; Dale, C.S.; Demarchi, D.S.; Gonçalves, L.R. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010, 55, 227–234.
- Fernandes, C.M.; Teixeira, P.C.F.; Leite, A.C.; Gutiérrez, J.M.; Rocha, F.A. The snake venom metalloproteinase BaP1 induces joint hypernociception through TNF-α and PGE2-dependent mechanisms. Br. J. Pharmacol. 2007, 151, 1254–1261.
- Bernardes, C.P.; Menaldo, D.L.; Mamede, C.C.; Zoccal, K.F.; Cintra, A.C.; Faccioli, L.H.; Stanziola, L.; de Oliveira, F.; Sampaio, S.V. Evaluation of the local inflammatory events induced by BpirMP, a metalloproteinase from Bothrops pirajai venom. Mol. Immunol. 2015, 68, 456–464.
- De Toni, L.G.; Menaldo, D.L.; Cintra, A.C.; Figueiredo, M.J.; de Souza, A.R.; Maximiano, W.M.; Jamur, M.C.; Souza, G.E.; Sampaio, S.V. Inflammatory mediators involved in the paw edema and hyperalgesia induced by Batroxase, a metalloproteinase isolated from Bothrops atrox snake venom. Int. Immunopharmacol. 2015, 28, 199–207.
- Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins 2021, 13, 868.
- Zambelli, V.O.; Picolo, G.; Fernandes, C.A.H.; Fontes, M.R.M.; Cury, Y. Secreted Phospholipases A2 from Animal Venoms in Pain and Analgesia. Toxins 2017, 9, 406.
- Mamede, C.C.; de Sousa, B.B.; Pereira, D.F.; Matias, M.S.; de Queiroz, M.R.; de Morais, N.C.; Vieira, S.A.; Stanziola, L.; de Oliveira, F. Comparative analysis of local effects caused by Bothrops alternatus and Bothrops moojeni snake venoms: Enzymatic contributions and inflammatory modulations. Toxicon 2016, 117, 37–45.
- Zhang, C.; Medzihradszky, K.F.; Sánchez, E.E.; Basbaum, A.I.; Julius, D. Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc. Natl. Acad. Sci. USA 2017, 114, E2524–E2532.
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14140–14145.
- Quindlen-Hotek, J.C.; Kartha, S.; Winkelstein, B.A. Immediate inhibition of spinal secretory phospholipase A2 prevents the pain and elevated spinal neuronal hyperexcitability and neuroimmune regulatory genes that develop with nerve root compression. Neuroreport 2020, 31, 1084–1089.
- Kokotos, G.; Six, D.A.; Loukas, V.; Smith, T.; Constantinou-Kokotou, V.; Hadjipavlou-Litina, D.; Kotsovolou, S.; Chiou, A.; Beltzner, C.C.; Dennis, E.A. Inhibition of group IVA cytosolic phospholipase A2 by novel 2-oxoamides in vitro, in cells, and in vivo. J. Med. Chem. 2004, 47, 3615–36128.
- Lam, D.K.; Schmidt, B.L. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. Pain 2010, 149, 263–272.
- Cattaruzza, F.; Amadesi, S.; Carlsson, J.F.; Murphy, J.E.; Lyo, V.; Kirkwood, K.; Cottrell, G.S.; Bogyo, M.; Knecht, W.; Bunnett, N.W. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br. J. Pharm. 2014, 171, 3814–3826.
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336.
- Li, X.; Tai, H.H. Thromboxane A2 receptor-mediated release of matrix metalloproteinase-1 (MMP-1) induces expression of monocyte chemoattractant protein-1 (MCP-1) by activation of protease-activated receptor 2 (PAR2) in A549 human lung adenocarcinoma cells. Mol. Carcinog. 2014, 53, 659–666.
- Quarch, V.; Brander, L.; Cioccari, L. An Unexpected Case of Black Mamba (Dendroaspis polylepis) Bite in Switzerland. Case Rep. Crit. Care. 2017, 2017, 5021924.
- Karlsson, E.; Mbugua, P.M.; Rodriguez-Ithurralde, D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. 1984, 79, 232–240.
- Wong, S.F.; Chung, F. Succinylcholine-associated postoperative myalgia. Anaesthesia 2000, 55, 144–152.
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284.
More
