Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Matthew Winans and Version 2 by Rita Xu.
Microbiology has long been a keystone in fermentation, and innovative yeast molecular biotechnology continues to represent a fruitful frontier in brewing science. Consequently, modern understanding of brewer’s yeast has undergone significant refinement over the last few decades.
- hybrid
- lager
- yeast
- introgression
- interspecific
- domestication
1. Species of Saccharomyces
Saccharomyces cerevisiae may be one of the oldest domesticated organisms known to humans. Domestication events imposed on brewing strains of the budding yeast species S. cerevisiae resulted in unique strains similar to the divergence seen in animal lineages of Canis familiaris breeds or the plant lineage of Brassica oleracea foods. It has been suggested that S. cerevisiae behaved as a synanthropic species, following human settlements as a commensal organism residing in gardens and vineyards, although the time period and location of the yeast’s origins has been the subject of much debate throughout history. Domesticated Saccharomyces brewing strains feature flocculation capabilities, fast fermentation rates, malt sugar utilization, pleasant aromas, and are largely negative for production of phenolic off flavors (POF) [1][2][1,2].
Nearly two centuries have passed since the first accessible description was produced regarding brewer’s yeast and its recognition in fermentation [3][4][3,4]. Recently, phylogenic research utilizing genomics and modern molecular biology techniques has shed some light on the historically convoluted nomenclature surrounding this budding yeast. Genomic analysis of the Saccharomyces genus has consolidated many variations into eight individual species: S. cerevisiae, S. paradoxus (syn. S. cariocanus, S. cerevisiae var. tetraspora, S. cerevisiae var. terrestris, S. douglasii), S. uvarum (syn. S. bayanus var. uvarum), S. mikatae, S. kudriavzevii, S. arboricola (syn. S. arboricolus), S. eubayanus, and S. jurei [3][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] (Table 1). Moreover, two natural hybrids are recognized in the Saccharomyces clade: S. pastorianus (syn. S. carlsbergensis, S. monacensis) and S. bayanus [20][21][22][20,21,22]. Most modern lager fermentations utilize S. patorianus yeasts.
Table 1. Current Saccharomyces Yeast Species History.
| Saccharomyces | Described | Substrate | Location | Reference | |
|---|---|---|---|---|---|
| cerevisiae | 1838 | Beer | Germany | [4] | |
| uvarum | 1898 | Ribes rubrum, redcurrant juice | South Holland, The Netherlands | [16][17] | [16,17] |
| paradoxus | 1914 | Tree sap | Russia | [15] | |
| kudriavzevii | 1991 | Decayed leaf | Japan | [18] | |
| mikatae | 1993 | Decayed leaf | Japan | [19] | |
| arboricola | 2008 | Fagaceae spp. | West China | [6] | |
| eubayanus | 2011 | Nothofagus spp. & parasitic fungi Cyttaria spp. | Andean, Patagonia | [11] | |
| jurei | 2017 | Quercus robur | Saint Auban, France | [14] |
2. Hybrid Nature of Yeast
Interspecific hybrids are not unique to lager brewing. For example, the livestock and agricultural industries commonly employ selective breeding to alter species’ properties or increase yields [23][24][25][23,24,25]. A time-honored showpiece of hybrid vigor is the mule, a great pack animal known for its hardiness and longevity. For over 4000 years, the mule has been bred as the hybrid progeny of a male donkey and a female horse. Since the early 1900s, maize has been hybridized to increase yields and introduce biodiversity [26]. Similarly, hybrid yeasts have been isolated from fermentation processes on numerous occasions [27] (Figure 1). A hybrid between S. cerevisiae and S. kudriavzevii was isolated from Belgian Trappist beers [28]. Popular in wine production, strain VIN7 is a hybrid of S. cerevisiae and S. kudriavzevii [29]. Other interspecific S. cerevisiae and S. uvarum hybrids are also regularly used for production of wines [29][30][29,30]. Spontaneous fermentations have yielded Pichia apotheca, a hybrid of P. membranifaciens and an unknown species [31].
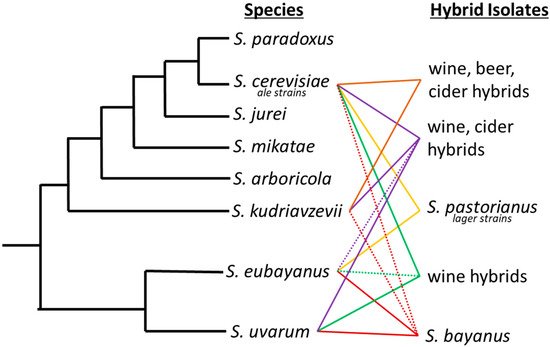
Figure 1. Saccharomyces phylogenetic tree with industrially important hybrids. Industrial hybrids are listed to the right by the fermentation they have predominately been associated with. Solid lines between two species signifies the interspecific hybrids and dashed lines denote introgression from a third or fourth species that may not always be present in each hybrid strain.
The mule of the brewing industry is the lager yeast S. pastorianus, an interspecific hybrid that produces the lion’s share, in volume, of the global beer production. Although its use is widespread, the biodiversity is limited to two main lineages, Saaz/group I (syn. S. carlsbergensis, (L12: Noble, Imperial Yeast Culture Collection, type strain CBS1513) and Frohberg/group II (L13: Global, Imperial Yeast Culture Collection, type strain-Weihenstephan 34/70). Saaz and Frohberg lineages vary in their genomic composition from each parent species, S. eubayanus and S. cerevisiae, which influences important fermentation characteristics. Genomic analysis demonstrated a genomic composition of 1:2 S. cerevisiae to S. eubayanus sub genome in the Saaz lineage and 2:2 S. cerevisiae to S. eubayanus sub genome in the Frohberg lineage supporting the traditional designations used by brewers [32][33][34][33,34,35].
3. Novel Hybrid Development
The first yeast breeding experiments aimed at combining desirable traits of brewing strains were conducted by Ojvind Winge during his tenure at the Carlsberg Laboratory in the 1930s [35][37]. Hybrid yeast development has been carried out for over half a century since then, aimed predominantly at increasing attenuation and fermentation rates via intraspecific crosses with ale and lab strains [36][37][38][38,39,40]. Modern fermentations benefit from many innate and acquired hybrids that have been isolated or developed [11][27][28][39][40][41][42][43][44][45][46][47][48][49][50][11,27,28,41,42,43,44,45,46,47,48,49,50,51,52]. Early efforts in brewing science established the fundamentals necessary to explore the phylogeny, genomics, and strain development for Saccharomyces fermentation. During typical rich nutrient propagations of yeast in a brewing environment, mother cells reproduce asexually to bud off small daughter clones (Figure 2). Under poor nitrogen conditions, such as proline, yeast growth changes to a pseudohyphal form [51][52][53,54]. The complete absence of a nitrogen source and the presence of a non-fermentable carbon source, such as acetate, will sporulate yeast cells [53][55]. Sporulation transforms the cell wall into the ascus, or sack, that holds four spores termed a tetrad. Analogous to the human egg and sperm, these spores divide equally into mating types as either a or α [54][56]. When conditions improve for yeast growth, new haploid (1n) yeast can conjugate with the opposite mating type yeast as they form a shmoo.
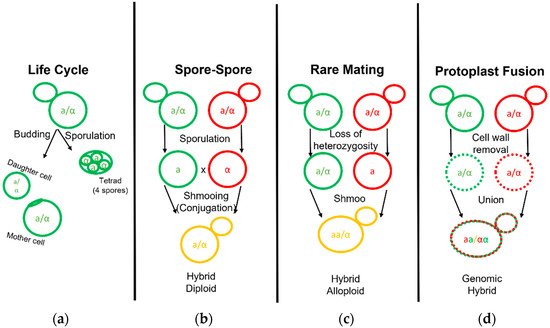
Figure 2. Life and Mating of Saccharomyces Yeast. Diagram pertaining to the clonal growth typical of yeast fermentation cultures and the various known techniques employed to generate yeast hybrids. (a) Diploid yeast cells may bud and grow clonally to form a mother and daughter cell or undergo sporulation to form a tetrad. (b) Yeast hybridization may form by direct spore to spore mating. (c) Yeast hybridization may form by rare mating events in which one or both diploid parent cells gain competency by becoming hemizygous or MATa/MATa and MATα/MATα diploids. (d) Yeast hybridization may also form by fusion of two separate yeast cell protoplasts with their cell wall removed.
Interspecific hybridization is seen as a valuable tool for yeast strain development, enabling the combination and enhancement of characteristics from both parental strains or species [55][57]. The development of hybrids is executed via three primary methodologies: spore–spore mating, rare mating, and protoplast fusion (Figure 2). Spore to spore mating is most similar to what would be considered natural mating, as outlined in Figure 2b. This approach bears a high success rate, high genomic stability, and can avoid the aid of selection markers such as drug resistance or autotrophies. Rare mating utilizes a described spontaneous loss of heterozygosity at the mating type locus. Normal diploid cells carry two sets of chromosomes with both the MATa and MATα genetic alleles and do not respond to sex pheromones for mating purposes. The spontaneous loss of either sex allele tolerates yeast mating to a yeast cell of complimentary sex. This results in yeast with high chromosome counts, influencing gene dosage during cellular processes and partially explains the outperformance over a diploid yeast of the same background [55][57]. Rare mating, as the name implies, is uncommon and selection markers are needed to perform this technique. The frequency of rare mating is estimated to occur in 1 out of 10 million cells [56][58]. This procedure is beneficial in overcoming poor sporulation but produces hybrids prone to high genomic instability. Lastly, protoplast fusion is performed by removing the cell wall and fusing the protoplasts of two cells together before the cell wall is repaired. This technique generates cells with a high chromosome copy number and higher genomic instability but overcomes low sporulation. This technique can be used in the laboratory to combine yeast from different genera such as the brewer’s yeast S. cerevisiae and other yeast outside of the Saccharomyces genera which are otherwise incompatible [57][59]. Protoplast fusion is considered genetic engineering in many parts of the world. Recent select investigations into Saccharomyces hybrid application in beverage fermentations are listed in table format (Table 2).
Utilizing advanced molecular biology techniques for the modification of yeast strains presents an ongoing endeavor by many academic labs and some commercial yeast laboratories. Several research groups have developed protocols that use plasmids carrying genetic markers for drug resistance or functional enzymes that target the mating system in yeast. Industrial strains are well-known for their poor ability to adhere to laboratory techniques including sporulation and transformation [58][60]. One strategy developed at the University of Wisconsin generates allotetraploid strains of prototrophic yeast without the need for sporulation or modification to the nuclear genome of parental yeast strains [59][61]. This method leverages a series of inducible plasmids coined HyPr (Hybrid Production) containing compilatory drug markers and an inducible HO cassette. HO encodes an endonuclease that performs a double stranded break in the DNA that determines the Saccharomyces sex type. To repair this damage, the yeast cell will copy the silenced sex type already present in the genome, effectively performing a sex change and allowing the cell to mate with other cells of the opposite mating type. Using drug resistance markers and the HO cassette, hybrids are produced and serial growth in the absence of drug selection purges cells of exogenous DNA [59][61].
Currently, the options for strain selection in S. cerevisiae yeast are plentiful, but the criteria for fermentation performance in the brewing environment remains selective. Strains are employed largely by beer style, equipment availability, and supporting knowledge base. Certain beer styles also contain defining features from specific yeast flavor active molecules [60][64]. Banana and clove flavors are derived from isoamyl acetate and 4-vinyl guaiacol (4VG) in Weissbier [60][61][62][64,65,66]. Hybridization of yeast bears several advantages in brewing to include transgressive phenotypes such as increased ethanolic fermentation performance or stress tolerance, shifting fermentation temperatures beyond traditional inhibitory conditions, and creating a mosaic blend of parental fermentation profiles [24][49][55][63][64][24,51,57,63,67]. Targets of yeast hybridization may include increased formation of glycerol for an enhanced mouthfeel, alternative carbon source metabolic ability, reduced off-flavor production, increased formation of antioxidants that increase beer flavor stability, or increase production of yeast longevity molecules such as trehalose. The methodologies to create yeast hybrids vary in their specificity to target genetic or phenotypic results, but efforts to harness yeast hybrids in brewing broadly increase the biodiversity of fermentation yeast, add depth to the complexity of fermentation profiles, and advance brewing science knowledge.
Table 2. Interspecific Yeast Hybrids in Fermentation.
| History | Parents | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| Isolated | S. cerevisiae × S. eubayanus | [11] | ||||||
| Isolated | S. cerevisiae ×S. eubayanus × | S. cerevisiae | S. uvarum | × S. eubayanus × S. uvarum | [39] | [41] | ||
| Isolated | S. cerevisiae × S. uvarum | [65] | [68] | |||||
| Isolated | S. cerevisiae × S. kudriavzevii | [27][28][40] | [27,28,42] | |||||
| Isolated | S. uvarum × S. eubayanus | [27][40] | [27,42] | |||||
| Developed | S. cerevisiae × S. eubayanus | [41][43]] | [43 | [44 | ,45 | ][45][66 | ,46,47,69] | |
| Developed | S. cerevisiae × S. mikatae | [46] | [48] | |||||
| Developed | S. cerevisiae ×S. kudriavzevii × | S. cerevisiae | × S. kudriavzevii | S. paradoxus | × S. paradoxus | [47] | [49] | |
| Developed | S. cerevisiae × S. kudriavzevii | [50] | [52] | |||||
| Developed | S. cerevisiae × S. arboricola | [46][49] | [48,51] | |||||
| Developed | S. cerevisiae × S. jurei | [67] | [70] |
