CoppTher is a metal that presents several industrial applic mining industry has faced significant challenges to maintaining copper production technically, economically, and environmentally viable. Some of the major limitations due to its attractive properties, such as electrical conductivity, thermal conductibility, and ductility. It is mainly used in electrical wiring, industrial machinery, plastic electroplating, printed wiring boards, zinc die casting, automotive bumpers, and rotogravure rolls. Copper is especially important in the semiconductor industry because, in general, that must be overcome in the coming years are the copper ore grade decline due to its intense exploitation, the increasing requirements for environmental protection, and the need to expand and construct new tailings dams. Furthermore, the risk of a supply crisis of critical metals, such as antimony and bismuth, has prompted efforts to increase their extraction from secondary resources in copper production. Therefore, improving conventional processes and developing new technologies is crucial to satisfying the world’s metal demands, while respecting the policies of environmental organizations. Hence, it is essential that the connections on chips are made of this metalhemical composition of each copper production stage is known for conducting these studies, which may be challenging due to its low electrical resistance, good mechanical properties, and high corrosion resistance. In addition to this, copper can form alloys with various elementthe huge variability of concentration data concerning the ore extraction region, the process type, and the operational conditions.
1. Introduction
Copper is a metal that presents several industrial applications due to its attractive properties, such as electrical conductivity, thermal conductibility, and ductility. It is mainly used in electrical wiring, industrial machinery, plastic electroplating, printed wiring boards, zinc die casting, automotive bumpers, and rotogravure rolls [1][2][1,2]. Copper is especially important in the semiconductor industry because, in general, the connections on chips are made of this metal due to its low electrical resistance, good mechanical properties, and high corrosion resistance [3]. In addition to this, copper can form alloys with various elements.
Copper is found in nature mainly in the form of sulfide and oxide minerals, and the main copper mines are present in Chile, Australia, Peru, China, the USA, and Mexico
[4]. In Chile, the world’s largest copper producer, most of the copper is obtained from sulfide ores
[5][6][5,6]. Since copper sulfide ores present impurities, several purification steps are carried out to obtain a high-purity copper product.
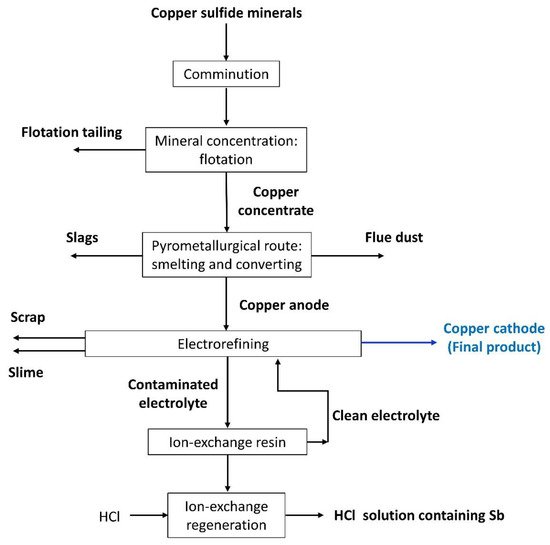
Figure 1 shows a simplified representation of copper production from sulfide minerals. Note that the ore is submitted basically to comminution, concentration, casting, and electrorefining stages
[7][8][7,8].
Figure 1.
Flowchart of the copper production from sulfide minerals showing the inputs and outputs of the main process stages.
The comminution stage aims to reduce the ore particle size through crushing, grinding, or other processes, being an energy-intensive operation with very low efficiency; it is estimated that comminution accounts for 30%–50% of typical mining operating costs
[9][10][9,10]. This is expected to intensify in the coming years due to the lower ore grades, the increase in rock hardness, and the depth of the mines. Therefore, researchers have been evaluating methods to reduce the energy consumption in the comminution process of copper ores
[9][11][12][13][9,11,12,13].
The copper concentration stage is usually conducted via flotation to separate the valuable minerals from the gangue material. The most important factors that affect the flotation performance are the ore characteristics, such as its mineral structure and the surface properties of the particle, the mechanical parameters of the cell, and the operating conditions, such as the solution pH
[14][15][14,15]. One of the major concerns regarding flotation is the generation of tailings, which is the waste material that may still contain copper and other valuable metals. It is expected that the tailings production will increase in the coming years due to the intense extraction of low-grade copper ores
[16]. This may limit the copper production in Chile due to the scarcity of areas available to expand tailings dams or construct new ones
[5]. Hence, authors have been evaluating methods to improve flotation processes, reduce and reprocess fresh and old tailings
[17][18][19][20][21][17,18,19,20,21].
The pyrometallurgical route involves smelting, converting and fire refining steps, which aim to separate the metal from its minerals and obtain copper anode. In the smelting stage, the concentrated flotation product (copper concentrate) is converted to molten high-Cu sulfide matte at temperatures of 1200–1300 °C
[7]. In the converting stage, air is injected into the liquid phase, which is composed basically of copper and iron sulfides; the iron sulfide is oxidized and the copper sulfide is converted into crude molten copper (~99%)
[22]. In the fire refining stage, the remaining sulfur and oxygen in the blister copper are eliminated through oxidation; sulfur is removed by adding O
2 (air), which reacts with S to form SO
2, whereas CO and H
2 are added to reduce Cu
2O and form Cu, CO
2 and H
2O
[7]. Thus, the fire-refined copper complies with the chemical specifications to be electro-refined. In these processes, large amounts of waste are generated, especially flue dust and slags
[23]. As flue dust contains copper with several impurities, such as As, Sb, Bi, Pb, and Cd, it cannot be returned directly to the smelting furnace since this would increase the content of impurities in the feed material and decrease the furnace processing capability
[24][25][24,25]. The formation of huge amounts of slags generates technical and environmental problems since they are deposited at landfills
[26]. Therefore, researchers have been evaluating the minimization of dust and slag formation, the recovery of copper, and the extraction of valuable metals from these waste materials
[22][27][28][29][22,27,28,29].
Lastly, the electrorefining stage aims at purifying the copper anode to generate the final copper product as a cathode. One of the major challenges in this process is controlling the concentration of metals in the tankhouse electrolyte that dissolve from the anode along with copper, such as arsenic, antimony, and bismuth. In general, the molar ratio of As/(Sb+Bi) is controlled and kept in a suitable range where Sb and Bi produce a minor effect. However, this increases health problems and the impurities are lost in the anode slime
[30][31][30,31]. Antimony and bismuth are valuable metals widely used in semiconductor, thermoelectric, pharmaceuticals, chemicals, ceramics, and pigments, also being considered as critical elements by the European Commission
[32][33][32,33]. Thus, extracting and recovering these metals from copper production has become increasingly important. In recent years, several methods have been tested for removing these impurities from tankhouse electrolytes, such as chemical precipitation
[34][35][34,35], solvent extraction
[36][37][36,37], using activated carbon
[38][39][38,39], chemical leaching
[40], electrodialysis
[41], electrowinning
[32], and ion-exchange resins
[30][42][30,42]. Among these technologies, ion-exchange resins are used on industrial scale worldwide
[30].
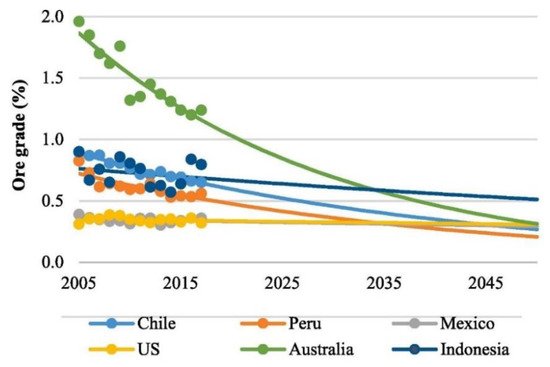
In the coming years, the mining industry will face a challenging scenario due to the gradual reduction in the mineral resources’ purity, while the global demand for copper and the stringency of environmental policies tend to increase considerably. The reduction in copper ore grade that occurred in the recent years and the projection for the coming years in Australia, Peru, Chile, Indonesia, Mexico, and USA may be seen in
Figure 2 [43]. This is particularly challenging for Chile since mining is the most important economic activity in the country
[44]. Lagos et al.
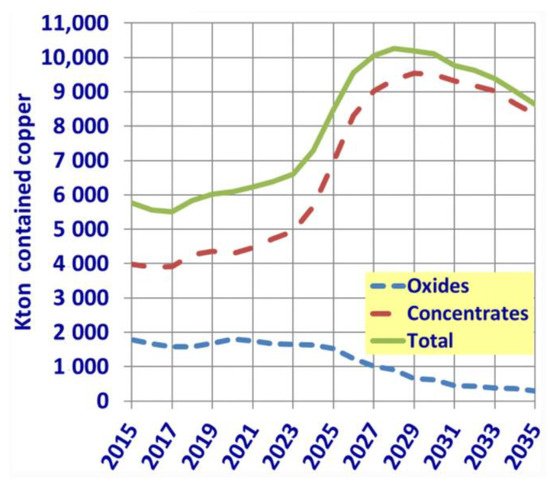
[5] have recently projected that, from 2030, copper production in Chile will decrease due to the lack of technologies that would make the extraction of low-grade ores feasible. This can be seen in
Figure 3, which shows the projection of copper production from oxide (blue line) and sulfide (red line—concentrates) ores obtained by Lagos et al.
[5] considering economic, regulatory, and environmental constraints. Similar trends were reported by Northey et al.
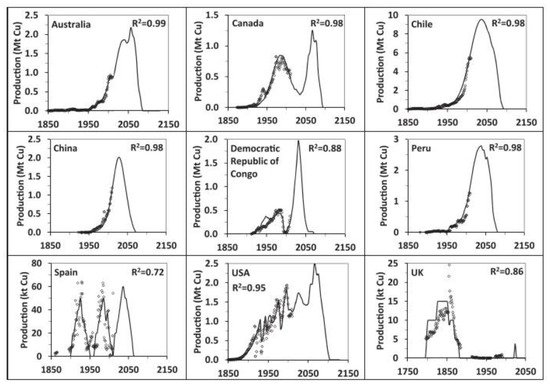
[45], who presented scenarios for copper production worldwide until 2150 based on a detailed assessment of global copper resources and historic mine production. The results for historic and modelled copper production by selected countries are shown in
Figure 4. Note that, by 2040, all countries will experience a strong reduction in their production, and this will be more critical for Chile because this country will be the largest copper producer before the decline in its production.
Figure 2.
Reported (symbols) and forecasted (lines) ore grade by country (Adapted from [43] with permission).
Figure 3. Projection of copper production in Chile in the coming years from oxide and sulfide (concentrate) ores (Adapted from
[5] with permission).
Figure 4.
Historic copper production data (symbols) and modelled (line) scenarios for selected countries [45].
Several studies have been carried out to improve Chilean copper production, reducing operational costs, reprocessing tailings, reducing losses of valuable metals, and making the processes safer for the environment and human health
[8][20][22][28][46][47][48][49][50][51][52][8,20,22,28,46,47,48,49,50,51,52]. For the development of these studies, knowing the chemical composition of each processing stage is essential; one of the limitations in the studies on copper production is the huge variability of concentration data depending on the region where copper is obtained, the process type, and the operational conditions. Thus, the knowledge of the chemical composition of each production stage may encourage the development of technologies to improve copper production and recover products from wastes, assisting the industrial supply of resources to feed the circular economy.
2. Copper Sulfide Minerals
Copper is found in nature mainly in oxide and sulfide ores. The oxide ores are processed via hydrometallurgical routes because they are easily dissolved in acid. Conversely, sulfide ores are practically insoluble in acid and must undergo pyrometallurgical routes
[40].
Table 1 shows the main copper minerals found in nature
[40][53][54][55][40,53,54,55].
Table 1.
Main copper minerals found in nature.
B. Note that pyrite accounts for 0.68% of the mineral, whereas chalcocite/digenite/covellite account for 0.42% and chalcopyrite/bornite 0.08%. Data on the mineralogical composition of copper oxide ores from Chile may be found elsewhere
[60].
Table 2.
Mineralogical composition of copper ore samples from Chile (wt%).
| A |
Chemical Composition (wt%) |
| Ref. |
[51] |
[61] |
[61] |
[61] |
[61] |
[57] |
[62] |
| Ref. |
Mine/region |
Cu |
Fe |
Mine |
Lomas Bayas |
El Salvador |
Chuquicamata |
Andina |
Escondida |
El Teniente |
Unknown |
| [64] |
Northern Chile |
1.49 |
10.36 |
Pyrite |
55.9 |
| [ | 38 |
65 | 35 |
6.2 |
30 |
|
|
| ] |
Cerro Colorado |
1.28–2.05 |
1.47–1.99 |
Chalcocite |
15.3 |
21 |
11.2 |
1.5 |
53 |
|
5.92 |
| [57] |
El Teniente |
1.20 |
|
Bornite |
11.3 |
1.51 |
1.65 |
0.27 |
0.11 |
6–9 |
18.85 |
| [7] |
Los Bronces |
1.06 |
Covellite |
7.9 |
14 |
17 |
1.1 |
0.6 |
|
0.71 |
| Muscovite/Sericite |
| 0.74 |
| |
| [7] |
Candelaria |
0.9–1.0 |
|
Chalcopyrite |
[ | 7.7 |
7.5 |
66 | 12 |
81 |
4.8 |
86–90 |
] |
Unknown |
0.7–0.86 |
| 74.51 |
| Digenite |
0.6 |
|
|
| B |
Chemical Composition (wt%) | |
|
|
|
| Enargite |
|
2.1 |
5.3 |
0.6 |
0.36 |
| Ref. |
Mine/region |
Cu |
Fe |
SiO2 | |
Al2O3 |
As | 0.01 |
| Pb |
Zn |
Ag |
S |
CaO |
Mg |
Au |
Molybdenite |
|
0.29 |
|
0.89 |
0.29 |
|
|
| [64 |
Metallic copper |
|
|
|
0.46 |
0.16 |
|
|
| Cuprite |
|
|
|
|
0.5 |
|
|
| Hematite |
|
|
|
0.2 |
0.08 |
|
|
| Others |
1.3 |
15.44 |
17.7 |
7.9 |
10.07 |
|
|
| B |
| ] |
Northern Chile |
1.49 |
10.36 |
48.09 |
8.6 |
<0.1 |
Antofagasta Region |
| 0.049 |
0.037 |
<5 |
2.26 |
8.07 |
2.92 |
<0.2 |
Ref. [63] |
| Pyrite |
0.68 |
Magnetite |
0.11 |
Kaolinite Group |
1.88 |
| Chalcocite/Digenite/Covellite |
0.42 |
Goethite |
0.01 |
Chalcopyrite/ Bornite |
0.08 |
Other Cu Minerals |
0.38 |
Chlorite/Biotite |
10.87 |
| Enargite/Tennantite/Tetrahedrite |
0 |
Other Fe Oxides/Sulfates |
0.26 |
Other Phyllosilicates |
0.92 |
| Native Cu/Cuprite/Tenorite |
0 |
Quartz |
24.44 |
Others |
0.53 |
| Molybdenite |
0.01 |
Feldspars |
58.66 |
|
|
| Oxides |
Cuprite |
Cu | 2 | O |
| Tenorite |
CuO |
| Malachite |
CuCO | 3 | ·Cu(OH) | 2 |
| Azurite |
(CuCO | 3 | ) | 2 | ·Cu(OH) | 2 |
| Chrysocolla |
CuO·SiO | 2 | ·2H | 2 | O |
| Atacamite |
Cu | 2 | Cl(OH) | 3 |
| Sulfides |
Chalcocite |
Cu | 2 | S |
| Covellite |
CuS |
| Chalcopyrite |
CuFeS | 2 |
| Bornite |
Cu | 5 | FeS | 4 |
| Stannite |
Cu | 2 | FeSnS | 4 |
| Enargite |
Cu | 3 | AsS | 4 |
| Tennantite |
Cu | 12 | As | 4 | S | 13 |
| Famatinite |
Cu | 3 | SbS | 4 |
| Tetrahedrite |
Cu | 12 | Sb | 4 | S | 13 |
About 80% of the world’s copper production comes from sulfide minerals, but their composition varies considerably depending on the region where the ore is obtained
[56].
Table 2A shows the mineralogical composition of copper sulfide ores from different Chilean regions. Note that the main copper mineral from Lomas Bayas, El Salvador, and Escondida mines is chalcocite (15.3%, 21% and 53%, respectively), whereas the main copper mineral from Chuquicamata mine, one of the largest copper producers in the world, is covellite (17%). The copper ores from El Teniente and Andina mines are mainly composed of chalcopyrite (86%–90% and 81%, respectively)
[57][58][57,58]. It is worth highlighting the presence of molybdenite in Chilean minerals, such as in El Salvador, Andina and Escondida mines, making Chile one of the world’s major producers of molybdenum
[59]. A recent and detailed characterization of copper sulfide ores from Antofagasta region, Chile, is shown in
Table 2
The chemical composition of Chilean copper sulfide ores in terms of Cu and Fe concentrations are shown in
Table 3A, whereas
Table 3B shows a more detailed chemical composition of copper sulfide ores from northern Chile. Note that the copper concentration varies between approximately 0.7 and 2.1 wt% depending on the region. Concentration data for other elements in copper ores, such as the critical metals, are scarce in the literature due to the difficulty in determining the composition of trace elements. Data on the chemical composition of oxide copper ores from Chile may also be found in the literature
[60].
Table 3.
Chemical composition of copper sulfide ores from Chile (wt%).