Depending on the appropriately tuned amplifiers and transceiver coils, in theory, any nuclear magnetic resonance (NMR) active nucleus can be used for imaging by MRI [24]. A nucleus with a spin quantum number of ½ (e.g., 1H, 3He, 13C, 14N, 15N, 19F, 19O, 31P, etc.) is designated to be in two spin states and the direction of spin alignment depends on the sign (+/−) of the gyromagnetic ratio, one of the two spin states will align along the magnetic field (ground state, lower energy), whereas the other one will align against it (excited state, higher energy). When an external magnetic field is applied, the spins in the ground state can be promoted to the excited state after absorbing the energy. Upon the termination of the external magnetic field, the spin returns to its equilibrium state (ground state) by a process called relaxation. There are two processes involved, each with an exponential time constant (Ti, i = 1,2): ‘T1’ (longitudinal or spin-lattice) or ‘T2’ (transverse or spin-spin) relaxation times. These parameters help in determining the signal/contrast-to-noise ratio (SNR) and the image resolution.
- magnetic resonance imaging
- nuclear magnetic resonace
- perfluorocarbons
- Gadolinium based contrast agents
1. Gadolinium Based Contrast Agents (GBCAs)
2. Fluorine as a Contrast Agent
3. Similarity between Fluorine and Hydrogen

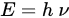

References
- Wahsner, J.; Gale, E.M.; Rodriguez-Rodriguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057.
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326.
- Index to Drug-Specific Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/index-drug-specific-information (accessed on 2 January 2022).
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28.
- Idee, J.M.; Port, M.; Raynal, I.; Schaefer, M.; Le Greneur, S.; Corot, C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: A review. Fundam. Clin. Pharmacol. 2006, 20, 563–576.
- Stockman, J.A. Oral Prednisolone for Preschool Children with Acute Virus-Induced Wheezing. Yearb. Pediatr. 2010, 2010, 515–518.
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565.
- Hequet, E.; Henoumont, C.; Djouana Kenfack, V.; Lemaur, V.; Lazzaroni, R.; Boutry, S.; Vander Elst, L.; Muller, R.N.; Laurent, S. Design, Characterization and Molecular Modeling of New Fluorinated Paramagnetic Contrast Agents for Dual 1H/19F MRI. Magnetochemistry 2020, 6, 8.
- Grobner, T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108.
- Yu, Y.B. Fluorinated dendrimers as imaging agents for 19F MRI. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 646–661.
- FDA Gadolinium Retention after Gadolinium Based Contrast Magnetic Resonance Imaging in Patients with Normal Renal Function; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017; pp. 127–131.
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568.
- Dekkers, I.A.; Roos, R.; van der Molen, A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2018, 28, 1579–1584.
- FDA. FDA Drug Safety Communication: FDA. In Warns that Gadolinium-Based Contrast Agents (GBCAs) are Retained in the Body; Requires New Class Warnings; FDA: Silver Spring, MR, USA, 2017.
- Hao, D.; Ai, T.; Goerner, F.; Hu, X.; Runge, V.M.; Tweedle, M. MRI contrast agents: Basic chemistry and safety. J. Magn. Reson. Imaging 2012, 36, 1060–1071.
- Knight, J.C.; Edwards, P.G.; Paisey, S.J. Fluorinated contrast agents for magnetic resonance imaging; a review of recent developments. RSC Adv. 2011, 1.
- Holland, G.N.; Bottomley, P.A.; Hinshaw, W.S. 19F magnetic resonance imaging. J. Magn. Reson. Imaging 1977, 28, 133–136.
- Weise, G.; Basse-Luesebrink, T.C.; Wessig, C.; Jakob, P.M.; Stoll, G. In vivo imaging of inflammation in the peripheral nervous system by 19F MRI. Exp. Neurol. 2011, 229, 494–501.
- Tirotta, I.; Dichiarante, V.; Pigliacelli, C.; Cavallo, G.; Terraneo, G.; Bombelli, F.B.; Metrangolo, P.; Resnati, G. 19F magnetic resonance imaging (MRI): From design of materials to clinical applications. Chem. Rev. 2015, 115, 1106–1129.
- Otake, Y.; Soutome, Y.; Hirata, K.; Ochi, H.; Bito, Y. Double-tuned radiofrequency coil for 19F and 1H imaging. Magn. Reson. Med. Sci. 2014, 13, 199–205.
- Keupp, J.; Rahmer, J.; Grasslin, I.; Mazurkewitz, P.C.; Schaeffter, T.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Simultaneous dual-nuclei imaging for motion corrected detection and quantification of 19F imaging agents. Magn. Reson. Med. 2011, 66, 1116–1122.
- Wolters, M.; Mohades, S.G.; Hackeng, T.M.; Post, M.J.; Kooi, M.E.; Backes, W.H. Clinical Perspectives of Hybrid Proton-Fluorine Magnetic Resonance Imaging and Spectroscopy. Investig. Radiol. 2013, 48, 341–350.
- Bouvain, P.; Temme, S.; Flogel, U. Hot spot 19 F magnetic resonance imaging of inflammation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1639.
- Liu, W.; Frank, J.A. Detection and quantification of magnetically labeled cells by cellular MRI. Eur. J. Radiol. 2009, 70, 258–264.
- Grapentin, C.; Barnert, S.; Schubert, R. Monitoring the Stability of Perfluorocarbon Nanoemulsions by Cryo-TEM Image Analysis and Dynamic Light Scattering. PLoS ONE 2015, 10, e0130674.
- Vatsadze, S.Z.; Eremina, O.E.; Veselova, I.A.; Kalmykov, S.N.; Nenajdenko, V.G. 18F-Labelled catecholamine type radiopharmaceuticals in the diagnosis of neurodegenerative diseases and neuroendocrine tumours: Approaches to synthesis and development prospects. Russ. Chem. Rev. 2018, 87, 350–373.
- Harris, R.K.; Becker, E.D.; de Menezes, C.; Sonia, M.; Goodfellow, R.; Granger, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818.
- Schmieder, A.H.; Caruthers, S.D.; Keupp, J.; Wickline, S.A.; Lanza, G.M. Recent Advances in 19Fluorine Magnetic Resonance Imaging with Perfluorocarbon Emulsions. Engineering 2015, 1, 475–489.
- Ruiz-Cabello, J.; Barnett, B.P.; Bottomley, P.A.; Bulte, J.W. Fluorine 19F MRS and MRI in biomedicine. NMR Biomed. 2011, 24, 114–129.
- Kissane, J.; Neutze, J.A.; Singh, H. Radiology Fundamentals, Introduction to Imaging & Technology, 6th ed.; Springer: Cham, Switzerland, 2020; 431p.
