Peripartum or perinatal depression, which is depression arising in the period between the start of a pregnancy and the end of the first postpartum year. Multiple CPGs recommend antidepressant initiation or continuation based on maternal disease severity, non-response to first-line non-pharmacological interventions, and after risk-benefit assessment. Advice on treatment of comorbid anxiety is largely missing or unspecific. Antidepressant dispensing data suggest general prescribers’ compliance with the preferred substances of the CPG, although country-specific differences were noted. To conclude, there is an urgent need for harmonized, up-to-date CPGs for pharmacological management of peripartum depression and comorbid anxiety in Europe. The recommendations need to be informed by the latest available evidence so that healthcare providers and women can make informed, evidence-based decisions about treatment choices.
- clinical practice guideline
- depression
- anxiety
- antidepressant
- psychotropic medications
- peripartum
1. Introduction
2. Treatment of Peripartum Depression with Antidepressants
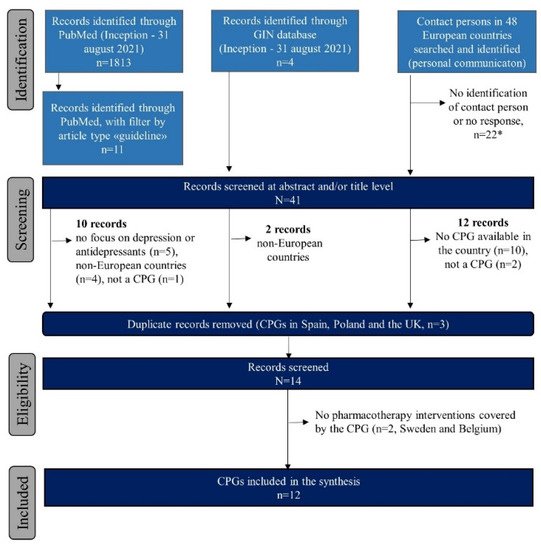
2.1. Identified Clinical Practice Guidelines
3.2. Pharmacological Interventions for Treatment of Antenatal Depression
| Country, Publication Year, Type | New Depression, Initiate AD | Preexisting Depression, Continue AD | AD Dose Adjustment and Monitoring | Switching AD | Preferred or Not Preferred AD |
AD Use before vs during Pregnancy (%) | Most Common ADs Used during Pregnancy | Treatment of Co-Morbid Anxiety | Other Psychotropics during Pregnancy (%) |
|---|---|---|---|---|---|---|---|---|---|
| Denmark [17] PMH-S |
2.3. Pharmacological Interventions for Treatment of Postpartum Depression
| Country, Publication Year, Type | Depression, Initiate or Continue AD | AD Intake by Time of BF | Switching AD | Preferred or Not Preferred AD | AD Use Postpartum (%) | Most Common ADs Postpartum | Treatment Co-Morbid Anxiety | Other Psychotropic Postpartum (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denmark [17 | Yes, if severe and no response to psychotherapy | ] PMH-SYes | NS | Yes, if effective for woman’s depression | NS | No, but weight gain in infant should be monitored. Formula can be considered. | No, if the AD is effective and was taken in pregnancy | Sertraline, paroxetine / Fluoxetine, (es)citalopram, fluvoxamine, venlafaxineSertraline, citalopram / Paroxetine, fluoxetine |
4.1 [29] | 2.0 vs. 1.9–4.1 [18][19] | 2.0 vs. 1.9–4.1 [18,19] |
Citalopram, sertraline, fluoxetine [19] | NS | BZD: 0.6 [20] AP: 0.4 [21 |
NA | NS | BZD: 1.3 [20] AP: NA Quetiapine: NA] Quetiapine: 0.2 [21] |
||||
| Finland [22] N-PPD | NS | Yes, in moderate-to-severe cases Psychotherapy first line |
|||||||||||||||||||
| Finland [22 | Monitoring of response is important | ] N-PPD | NS | As for non-pregnant adults, psychotherapy is recommended for mild symptoms | No, use of SSRI does not prevent BF | NS | SSRIs / FluoxetineSSRIs / Paroxetine, fluoxetine, tricyclics |
1.6–4.0 [23] vs. 3.6* [24] |
NA | NS | BZD: 1.2* [25] AP: 0.8 [21] Quetiapine: 0.9 |
NA[21] | |||||||||
| NA | NS | BZD: 0.7–3.2 [ | 47 | ] | AP: NA | Quetiapine: NA | Germany [26] N-PPD | Yes, after individual risk benefit evaluation, individual disease history, preference and availability of alternative treatments | Yes, in moderate-to-severe cases. Abrupt discontinuation is discouraged. Psychotherapy first line |
Monotherapy if possible, lowest effective dose Continuous measurement of plasma levels |
NS | Sertraline, citalopram / Paroxetine, fluoxetine |
4.0 [27] vs. 0.4 (unpublished data) |
Amitriptyline, (es)citalopram, sertraline (unpublished data) | NS | BZD: 3.3 [25] AP: 0.3 [ | |||||
| Germany [26] N-PPD | 21 | Yes, after risk–benefit analysis for mother and child and individual disease history, preference, and availability of alternative treatments | Yes, after risk–benefit analysis for mother and child | NS | SSRIs, tricyclics / NS |
NA | NA | NS | BZD: NA AP: NA ] Quetiapine: 0.2 [21] |
||||||||||||
| Quetiapine: NA | Italy [28] PMH-S |
Yes, after individual risk–benefit assessment | |||||||||||||||||||
| Italy [28 | Yes, after individual risk–benefit assessment | ] PMH-S | NS | NS | NS | Yes, after risk–benefit analysis for mother and child | No, use of SSRI does not prevent BF | NS | NS / Fluoxetine |
2.5–3.4 [29]3.3–4.4 [29] vs. 1.2–1.6 [29] |
Paroxetine, sertraline, citalopram [29] | Yes, BZD can be used | NA | Yes, short-term acting BZD | BZD: NA AP: NA BZD: 1.4 [30] AP: 0.8 [31] Quetiapine: NA SAH: 0.4* [24] |
||||||
| Quetiapine: NA | Malta [32] PMH-S |
Yes, after individual risk–benefit assessment; drug choice based on lowest risk, monotherapy if possible and at the lowest effective dose | Yes, after individual risk-benefit assessment; drug choice based on lowest risk, monotherapy if possible and at the lowest effective dose; previous response is considered | NS | If possible, switch paroxetine to other SSRI | Sertraline ¥; Fluoxetine / Paroxetine |
NA | Sertraline, fluoxetine (unpublished data) | BZD only short term for extreme anxiety or agitation; BZD should be avoided in late pregnancy | BZD: NA AP: NA Quetiapine: NA |
|||||||||||
| Latvia [48] PMH-S |
Yes, after risk–benefit analysis for mother and child in case of BF. For initiation of AD, start with lowest effective dose. | Assess whether dosage and regimen are compatible with BF | NS | SSRIs, sertraline / Fluoxetine |
NA | NA | Yes, mirtazapine or atypical AP; quetiapine for augmentation therapy. Olanzapine only at low doses. BZD should be avoided. | BZD: NA AP: NA Quetiapine: NA |
The Netherlands [33] PMH-S |
||||||||||||
| Malta [32] | Yes, after individual risk–benefit assessment | Yes, if woman is stable with medication | Yes, lowest effective dose; paroxetine preferably not >20 mg/day | If possible, switch paroxetine to other SSRI but pre-pregnancy | Sertraline ¥ / Paroxetine ¥ |
3.9 vs. 2.1 [34] |
PMH-SSertraline, paroxetine, citalopram [ |
Yes, after individual risk–benefit assessment; drug choice based on lowest risk, monotherapy if possible and at the lowest effective dose34 | Yes, after individual risk–benefit assessment; drug choice based on lowest risk, monotherapy if possible, and at the lowest effective dose, previous response is considered | NS | Iimipramine, nortriptyline, sertraline / Citalopram, fluoxetine] |
NS | BZD: 1.1 [25][35] AP: NA Quetiapine: NA | BZD: 1.1 [25,35] AP: NA |
NA Quetiapine: NA |
||||||
| SSRIs e.g., sertraline, paroxetine (unpublished data) | Short-term BZD (caution in BF). Close monitoring of babies exposed to BZD via breastmilk. Diazepam should not be used while BF. | BZD: NA | AP: NA | Quetiapine: NA | Norway [36] PMH-S | Yes, if severe and with non-pharmacological therapy |
Yes, after individual risk–benefit assessment. Psychotherapy first line. Abrupt discontinuation is discouraged. |
Yes, serum concentration | No | Choice based on prior drug response and its safety profile | |||||||||||
| The Netherlands 33 PMH-S |
Yes, continue SSRI after delivery | Yes, BF is recommended | No, no evidence for switching | Paroxetine, sertraline / Fluoxetine, citalopram |
3.1 [34] | 2.0 vs. 1.5 [37] |
Paroxetine, citalopram,(Es)citalopram, sertraline, venlafaxine [19] |
NS | sertraline [34] |
NS | BZD: NA AP: NA Quetiapine: NABZD: 0.9 [38] AP: 1.2 [21] Quetiapine: 0.3 [21] SAH: 1.0 [24] * |
||||||||||
| Poland [39] PMH-S |
Individual risk–benefit assessment to be made. **AD in 1 trimester should be avoided, and AD should be discontinued before delivery |
If severe depression or ongoing mild-to-moderate symptoms, AD should be considered. Gradual discontinuation if mild symptoms with psychotherapy. ** As for new depression. |
Monotherapy, lowest effective dose | Yes, switching an AD which is effective and offers fewer adverse effects | NS / Paroxetine |
- vs. 0.3 [24] * | NA | Yes, but do not offer BZD except for the short-term treatment of severe anxiety and agitation | |||||||||||||
| Norway [36] PMH-S | BZD: 0.2–14.0 | [ | 24 | ] | [ | 25 | ] | * | AP: NA Quetiapine: NA SAH: 0.4 [24] * | BZD: 0.2–14.0 [24,25] * AP: NA Quetiapine: NA SAH: 0.4 [24] * |
|||||||||||
| Yes, if severe after individual risk–benefit assessment | NS | No | Sertraline, paroxetine | / Doxepin, fluoxetine, citalopram |
1.0 [37] | NA | NS | BZD: 0.8 [37] AP: 0.2 [37] Quetiapine: NA |
Serbia [40] N-PPD |
Yes, if severe after individual risk–benefit assessment |
Yes, after individual risk–benefit assessment | Yes, serum concentration | NS | Fluoxetine, | [25 | ||||||
| Poland [39] PMH-S | citalopram, | fluvoxamine, | paroxetine, sertraline / TCA |
- vs. 0.3 [24] * | (Es)citalopram, sertraline, mirtazapine, duloxetine (unpublished data) |
Yes, | Yes, initiate if severe and continue to prevent relapse. If history of severe depression or ongoing mild-to-moderate symptoms, AD should be considered. |
Yes, AD in one daily dose before the child’s longest sleep, and BF is recommended just before AD intake |
No, same treatment pattern should be used after delivery | ] | Sertraline, citalopram / Fluoxetine BZD |
BZD: 0.2–14.0 [24 * | NA | ] |
NA | NA | BZD: NA AP: NA Quetiapine: NA | AP: NA Quetiapine: NA SAH: 0.4 [24] * | BZD: 0.2–14.0 [24,25] * AP: NA Quetiapine: NA SAH: 0.4 [24] * |
||
| Spain [41] PMH-S |
|||||||||||||||||||||
| Serbia [ | Yes, after individual risk–benefit assessment | 40] N-PPDYes, after individual risk–benefit assessment and based on individual drug response |
Monotherapy if possible, lowest effective dose; continuous measurement of plasma levels due to fluctuations in pregnancy is recommended | Yes, if lower risk to child and effective in mothers | SSRIs | Yes, if severe after individual risk–benefit assessment / Paroxetine, tricyclics, fluoxetine |
- vs. 0.5–0.8 [ |
NS | 42 | No | ] | Fluoxetine | [43] | - vs. 0.5–0.8 [42 |
NA,43] | Paroxetine, citalopram, fluoxetine44 | Yes, but for acute symptoms for maximum 4 weeks | BZD: 1.9 [42] AP: 0.1 [43] Quetiapine: NA |
|||
| Paroxetine | (data unpublished) | NS | BZD: NA | AP: NA | Quetiapine: NA | United Kingdom [44][45] PMH-S | United Kingdom [44,45] PMH-S |
Yes, particularly for moderate-to-severe depression, after discussing with the woman the risk–benefit assessment of AD; drug choice based on lowest risk, monotherapy if possible and at the lowest effective dose |
Yes, particularly for moderate-to-severe depression, after discussing with the woman the risk–benefit assessment of AD; monotherapy if possible and at the lowest effective dose | Yes, dosages may need to be adjusted in pregnancy | |||||||||||
| Spain [ | Option to be discussed with the woman but aim is to expose fetus to as few drugs as possible | 41] PMH-S |
Yes, if severe after individual risk–benefit assessment | NS | NS | Nortriptyline, sertraline, paroxetine / Citalopram, fluoxetineUnspecified, choice based on prior drug response and its safety profile |
8.8–9.6 vs. 3.7 [29] |
Fluoxetine, citalopram [29] | Yes, with ADs. Do not offer BZD except for the short-term treatment of severe anxiety and agitation. | NA | NA | NS | BZD: NA AP: NA Quetiapine: NA | BZD: 1.2 * [25] AP: 0.3–4.6 [21][46] Quetiapine: 0.4 [21] | BZD: 1.2 * [25] AP: 0.3–4.6 [21,46] Quetiapine: 0.4 [21] |
| United Kingdom | |||||||||
| [ | |||||||||
| 44 | |||||||||
| ] | |||||||||
| [ | |||||||||
| 45 | |||||||||
| ] | |||||||||
| PMH-S | |||||||||
| United Kingdom | |||||||||
[44,45] PMH-S |
Yes, particularly for moderate-to-severe depression after discussing with the woman of the risk–benefit assessment of AD; drug choice based on lowest risk, monotherapy if possible and at the lowest effective dose. | Consider risks and benefits of BF, which should generally be encouraged, but monitor baby for any adverse effects. | Option to be discussed with the woman, but aim is to expose the breastfed infant to as few drugs as possible. | Unspecified, choice based on prior drug response and its safety profile in breastfeeding. | 5.5–12.9 [29][46] | 5.5–12.9 [29,46] | SSRI [49] | Yes, but do not offer BZD except for the short-term treatment of severe anxiety. BZD best avoided in BF if possible; use drug with shortest half-life. | BZD: NA AP: 0.4 [46] Quetiapine: NA |
