Doublecortin (DCX) is an microtubule associatedessential protein, essential for correctin in the development of the central nervous system development andand in lamination inof the mammalian cortex. It has been demonstrated to bis known that the expressed in developing but not in matureion of DCX is restricted to newborn neurons. The visual system of teleost visual system ifish has been postulated as an ideal model to study mechanisms of adult neurogenesis due to its continuous life-long growth. Immunohistochemical, in silico,since it continuously grows throughout the animal’s life. Here, we report a comparative expression analysis of DCX between two teleost fish species ands western blotll as a bioinformatic analysis to detect the DCX proteinwith other animal groups. Our results demonstrate that DCX is very useful for identifying new neurons in the visual systems of Astatotilapia burtoni, buteleost fish are described is absent in hereDanio rerio.
1. Introduction
Doublecortin (DCX; also known as doublin or lissencephalin-X) is a microtubule-associated protein which is typically expressed in the early neuronal differentiation stage, both in precursors and immature neurons
[1][2][1,2]. Due to DCX expression being nearly exclusive to developing neurons, several research groups are using it as a marker for neurogenesis in a wide range of vertebrate species, e.g., mammals
[3], lampreys
[4], sharks
[5], and teleosts
[6].
Brain formation depends on microtubules (MTs) and accompanying microtubule associated proteins (MAPs) to regulate specific migration of different neural cell types
[7]. Brain development includes nuclear displacement and process formation that require the action of MTs and specific MAPs
[8]. Furthermore, MTs are essential in the formation of growth cones
[9]. The de-stabilization of MTs leads to the collapse of the migrating cell body and cessation of nuclear translocation
[10]. Faulty of DCX expression causes critical brain defects, which implies that other MTs stabilizing proteins cannot compensate for DCX function in the central nervous system (CNS)
[7][11][7,11]. In newly formed neurons, DCX are involved in the growth of neuronal processes
[12][13][12,13].
The continuous life-long growth of the visual system of anamniotes (such as teleost fish) has been an intriguing phenomenon
[14][15][14,15], especially since such extensive growth does not occur in mammals
[16][17][16,17]. Several studies have used different fish species to understand the mechanism of adult neurogenesis in vertebrates
[2][18][19][2,18,19]. The retina has been proposed as an ideal model to study the generation of new neurons in adults due to the presence of well-delimited neurogenic zones
[20][21][20,21]: the peripheral germinal zone (PGZ), formed by stem cells; the transition zone, occupied by differentiating cells; and the layered retina, harboring completely differentiated cells, except for the generation of new rods which are added to the outer nuclear layer from rod precursors
[22]. Among the many markers used to label differentiating neurons, DCX stands out both in mammals and some teleosts such as cichlid fish
[6][23][6,23]. Given that commercially available anti-DCX-antibodies efficiently label processes from maturing neurons
[12],
rwe
searchers were interested in testing DCX as a potential marker to study differentiating neurons within the retinal transition zone of the fish retina.
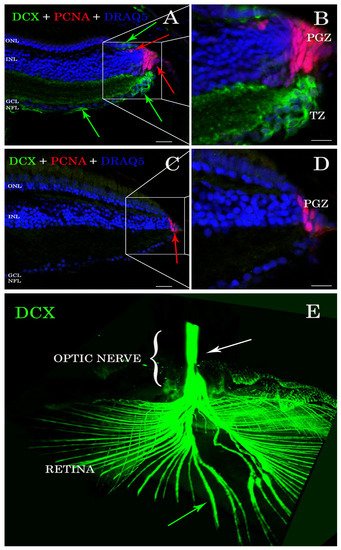
2. DCX Is Present in A. burtoni Retina but Not D. rerio
Antibody stains applied to histological sections enabled the detection of DCX expression in the
A. burtoni retina (
Figure 1A,B). In contrast, no specific immunofluorescence was found in the zebrafish retina (
Figure 1C,D). DCX positive cell bodies were located in the transition zone (
Figure 1A,B), next to the PGZ, of the retina of
A. burtoni and their axons passing through the nerve fiber layer to the optic nerve head (
Figure 1E). No cell bodies expressing DCX were observed in the differentiated retina and/or in the optic nerve.
Figure 1. Sections of retina (A–D) and cleared tissue (E). DCX positive cells (green arrows) are detected in A. burtoni retina (A) but not in zebrafish (C). Magnification of (A,C) are presented in (B,D). The proliferating cell nuclear antigen (PCNA; red arrows) binding antibodies detect cells in the peripheral germinal zone (PGZ; of both species. In addition, DCX (green arrows) and PCNA (red arrows) are detected in the ONL corresponding to cones and rod precursors, respectively. Nuclei are stained with DRAQ5 (blue). No double positive cells for DCX (green arrows) and PCNA (red arrows) are found. By analyzing the whole transparent eyeball, it is possible to follow DCX positive processes in the neural fiber layer (green arrow) into the optic nerve (E; white arrow). Scale bar 20 µm (A,C); 10 µm (B,D). ONL: outer nuclear layer; INL: inner nuclear layer; GCL: ganglion cell layer; NFL: neural fiber layer.
3. DCX Proteins Are Present in All the Genomes Analyzed but Not in Zebrafish
ResearchWe
rs generated a database containing 159 protein sequences belonging to 14 teleost fish (
Triplophysa tibetana, Danio rerio, Betta splendens, Parambassis ranga, Oryzias latipes, Nothobranchius furzeri, Astatotilapia burtoni, Gadus morhua, Anabarilius grahami, Nothobranchius pienaari, Oryzias melastigma, Carassius auratus, Labeo rohita, and
Tetraodon nigroviridis), 6 mammals (
Mus musculus, Homos sapiens, Pan troglodytes, Canis lupus, Bos taurus, and
Rattus norvegicus), one bird (
Gallus gallus), and one shark (
Callorhinchus milii), as well as
D. melanogaster and
C. elegans as invertebrate species (
Table 1).
Table 1. Database of 159 amino acidic sequences selected to perform protein alignments. Different species are used to compare DCX and DCLK sequence identity. From this database rwesearchers also extract DCX1 and DCX2 conserved domains for further sequence analyses.
| Protein and Species |
Accession Number |
Protein and Species |
OrganismAccession Number |
Protein and Species |
Accession Number |
| Number of Aligned Sequences |
Similarity |
| DCLK from Kanglang fish |
ROJ66296 |
DCLK from Human |
NP_004725 |
| DCX |
Human + Shark |
15 | DCLK from Zebrafish |
AAI63926 |
| 96.7% |
DCLK from Kanglang fish |
ROL01462 |
DCLK from Human |
XP_016876336 |
DCLK from Zebrafish |
AAI68500 |
| Mammals |
47 |
89% |
DCLK from Kanglang fish |
ROL42582 |
| Mammals + A. burtoni fish | DCLK from Human |
XP_016876337 |
DCLK from Zebrafish |
BAF45322 |
| 48 |
88.8% |
). Remarkably, results revealed that the DCX2 domain is much more conserved than DCX1 and the kinase domains among species analyzed, and the latter, appears to be not much conserved in the evolutionary scale (
Table 3). Comparison of DCX1 and DCX2 domains of DCLK proteins from zebrafish and of DCX proteins from
A. burtoni fish revealed a high similarity of sequence and structure (
Table 3).
Table 3. Sequece similarities between DCX1 and DCX2 domains after its extraction from DCX and/or DCLK. Comparison of DCX1 and DCX2 conserved domains to analyze the identity between different species.
| Domain |
Organism |
Number of Sequences |
Similarity |
| DCX1 |
All |
159 |
73.4% |
| DCX2 |
All |
159 |
77.4% |
| DCLK from Kanglang fish |
| Kinase |
All |
159 |
ROL54251 |
DCLK from Medaka |
XP_004075345 |
DCLK from Zebrafish |
BAF45323 |
| DCLK from Betta fish |
| 47.4% |
Mammals + Teleost Fish |
58 |
83.3% |
XP_029027475 |
| DCX1 |
Teleost Fish |
79 |
74.5% |
DCLK from Medaka |
XP_011474963 |
| Teleost Fish |
11 | DCLK from Zebrafish |
BAF45324 |
| DCLK from Betta fish |
XP_029027480 |
DCLK from Medaka |
XP_011474968 |
DCLK from Zebrafish |
BAF45325 |
| 82.5% |
| Mammals + Teleost Fish + Drosophila + Shark + Chick |
61 |
78.6% |
DCLK from Betta fish |
XP_029027952 |
DCLK from Medaka |
XP_011474974 |
DCLK from Zebrafish |
BAF45326 |
| DCLK from Betta fish |
XP_029027953 |
DCLK from Medaka |
XP_011480649 |
DCLK from Zebrafish |
NP_001128593 |
| DCLK from Betta fish |
| DCX2 |
Teleost Fish |
79 |
79.1% |
| Kinase |
Teleost Fish |
79 |
69.9% |
DCLK |
| DCX1 | Mammals |
30 |
DCLK ZF + DCLK A. burtoni + DCX from another organisms |
97 |
79.6% |
XP_029027954 |
DCLK from Medaka |
XP_011481611 |
DCLK from Zebrafish |
NP_001139259 |
| DCLK from Betta fish |
XP_029027955 |
DCLK from Medaka |
XP_011485544 |
DCLK from Zebrafish |
NP_001139260 |
| DCLK from Betta fish |
XP_029030116 |
DCLK from Medaka |
XP_020563782 |
DCLK from Zebrafish |
NP_001139261 |
| DCLK from A. burtoni fish |
XP_005926513 |
DCLK from Medaka |
XP_020564443 |
| DCX2 |
DCLK ZF + DCLK A. burtoni + DCX from another organisms |
97 |
83.7% |
DCLK from Zebrafish |
XP_005172728 |
| DCLK from A. burtoni fish |
XP_005926514 |
DCLK from Medaka |
XP_024115177 |
DCLK from Zebrafish |
XP_009290868 |
| 55.9% |
| Teleost Fish |
71 |
DCLK from A. burtoni fish |
XP_005928154 |
DCLK from Medaka |
XP_024143878 |
DCLK from Zebrafish |
XP_009303718 |
| DCLK from A. burtoni fish |
XP_005935114 |
DCLK from Mouse |
AAH21354 |
DCLK from Zebrafish |
XP_009303720 |
| 53.5% |
DCLK from A. burtoni fish |
XP_005935115 |
DCLK from Mouse |
AAH50903 |
DCLK from Zebrafish |
XP_021325742 |
| DCLK from A. burtoni fish |
XP_005935116 |
DCLK from Mouse |
AAH64783 |
DCLK from Zebrafish |
XP_021334856 |
| DCLK from A. burtoni fish |
XP_005951753 |
DCLK from Mouse |
AAI33686 |
DCX from Betta fish |
XP_029029871 |
| DCLK from A. burtoni fish |
XP_005951754 |
DCLK from Mouse |
AF155819_1 |
DCX from Chick |
AF330009 |
| DCLK from A. burtoni fishA. burtoni fish |
XP_014190901 |
DCLK from Mouse |
NP_001182467 |
DCX from Dog |
XP_022271525 |
| DCLK from A. burtoni fish |
XP_014190902 |
DCLK from Mouse |
NP_001182468 |
DCX from Dog |
XP_022271526 |
| DCLK from A. burtoni fish |
XP_014190903 |
DCLK from Mouse |
NP_001344395 |
DCX from Dog |
XP_022271527 |
| DCLK from A. burtoni fish |
XP_014190904 |
DCLK from Mouse |
NP_001344397 |
DCX from Dog |
XP_022271528 |
| DCLK from A. burtoni fish |
XP_014190905 |
DCLK from Mouse |
NP_001344398 |
DCX from Dog |
XP_022271529 |
| DCLK from A. burtoni fish |
XP_014190906 |
DCLK from Mouse |
NP_001344404 |
DCX from Dog |
XP_022271530 |
| DCLK from A. burtoni fish |
XP_014190907 |
DCLK from Mouse |
NP_001344405 |
DCX from Dog |
XP_022271531 |
| DCLK from A. burtoni fish |
XP_014193189 |
DCLK from Mouse |
NP_064362 |
DCX from Dog |
XP_022271532 |
| DCLK from A. burtoni fish |
XP_014193190 |
DCLK from Mouse |
Q9JLM8 |
DCX from Fly |
AAM11416 |
| DCLK from A. burtoni fish |
XP_014193191 |
DCLK from Mouse |
XP_006501044 |
DCX from Glassy fish |
XP_028278320 |
| DCLK from Codfish |
XP_030217849 |
DCLK from Mouse |
XP_006501045 |
DCX from Glassy fish |
XP_028278321 |
| DCLK from Glassy fish |
XP_028258882 |
DCLK from Mouse |
XP_006501046 |
DCX from Glassy fish |
XP_028278322 |
| DCLK from Glassy fish |
XP_028258890 |
DCLK from Mouse |
XP_017174936 |
DCX from Human |
AAC31696 |
| DCLK from Glassy fish |
XP_028258898 |
DCLK from Mouse |
XP_030108271 |
DCX from Human |
AAC31797 |
| DCLK from Glassy fish |
XP_028258907 |
DCLK from Mouse |
XP_036018776 |
DCX from Human |
AAC52037 |
| DCLK from Glassy fish |
XP_028275059 |
DCLK from Rat |
NP_445795 |
DCX from Human |
AAH27925 |
| DCLK from Glassy fish |
XP_028275060 |
DCLK from Tibetan fish |
KAA0702164 |
DCX from Human |
CAA05867 |
| DCX from Rat |
| Mammals + Teleost Fish |
101 |
49.3% |
DCLK from Human |
NP_445831 |
| DCLK from zebrafish + DCX from D. melanogaster |
16 |
72.5% |
| DCLKs + DCX |
159 |
50.1% |
XP_014186335 |
DCLK from Mouse |
NP_001104521 |
DCX from Chimp |
PNI40040 |
| DCLK from A. burtoni fish |
XP_014190393 |
DCLK from Mouse |
NP_001104522 |
DCX from A. burtoni fish |
XP_005922256 |
| DCLK from A. burtoni fish |
XP_014190900 |
DCLK from Mouse |
NP_001104523 |
DCX from Cow |
NP_001193894 |
| DCLK from | AAI52457 |
DCLK from Tibetan fish |
KAA0704105 |
DCX from Human |
CAA06617 |
| DCLK from Human |
NP_001317000 |
DCLK from Tibetan fish |
KAA0710366 |
DCX from Human |
NP_001182482 |
| DCLK from Human |
NP_001317001 |
DCLK from Tibetan fish |
KAA0722200 |
DCX from Human |
NP_001356299 |
| DCX from Human |
NP_001356300 |
DCX from Mouse |
AAT58219 |
DCX from Rat |
AAG18479 |
| DCX from Human |
NP_001356301 |
DCX from Mouse |
AF155820_1 |
DCX from Human |
NP_835364 |
DCX from Mouse |
BAA33387 |
DCX from Rat |
Q9ESI7 |
| DCX from Human |
NP_835365 |
DCX from Mouse |
NP_001103692 |
DCX from Rat |
XP_006257444 |
| DCX from Human |
NP_835366 |
DCX from Mouse |
NP_001103693 |
DCX from Rat |
XP_006257447 |
| DCX from Human |
O43602 |
DCX from Mouse |
NP_001103694 |
DCX from Rat |
XP_006257448 |
| DCX from Killifish |
AEY83972 |
DCX from Mouse |
NP_034155 |
DCX from Rat |
XP_017457656 |
| DCX from Medaka |
XP_023818290 |
DCX from Mouse |
O88809 |
DCX from Shark |
AFP00992 |
| DCX from Mouse |
AAC31799 |
DCX from Mouse |
XP_006528761 |
DCX from Tibetan fish |
KAA0710823 |
| DCX from Mouse |
AAH56391 |
DCX from Mouse |
XP_030107072 |
Synapsin from Zebrafish |
BAH84839 |
| DCX from Mouse |
AAH57010 |
DCX from Mouse |
XP_030107073 |
|
|
| DCX from Mouse |
AAH62974 |
DCX from Mouse |
XP_030107074 |
Alignment of DCX amino acidic sequences showed a high conservation degree within and among analyzed groups (
Table 2). Interestingly, shark and human DCX homologues share 96% of similarity, but no DCX coding sequence was found in the genome of zebrafish.
Table 2. Sequence similarities between selected proteins containing DCX1 and DCX2 domains. Comparison of entire DCX and/or DCLK amino acid sequences to assess protein conservation between different species.
Since
rwe
searchers could not find DCX in zebrafish,
researcherswe also considered the DCLK proteins, which have been previously described in zebrafish, and belong to the DCX superfamily
[24][29]. DCLK sequences showed around 50–55% pairwise identity among species (
Table 2). No DCLK sequences were found in the genomes of invertebrate species (
D. melanogaster and
C. elegans). However, analysis of DCX proteins from
D. melanogaster revealed a catalytic “protein C kinase-like” domain (IPR000719, PF00069)
(Figure S2) located between residues 477–743. Based on these results,
rwe
searchers performed a multiple-sequence alignment of all the retrieved DCX and DCLK sequences. Results revealed more than 50% sequence similarity between DCX and DCLK sequences (
Table 2).
RWe
searchers then extracted the DCX1 conserved domain from DCX and DCLKs proteins, which was used to perform a multiple-sequence alignment. A similar analysis was conducted for DCX2 conserved domain. In both cases, a high conservation degree was observed between DCX and DCLKs proteins (
Table 3). The same approach was applied to the kinase domain found in DCLK proteins from vertebrates, using the “protein C kinase-like” catalytic domain of the
D. melanogaster DCX sequence as reference (Accession number: AAM11416)
(Figure S2
To verify the absence of DCX in the zebrafish genome,
reswe
archers performed a tblastn analysis using human DCX amino acidic sequence as query. Although no DCX orthologous genes were found on zebrafish,
researcherswe found three loci putatively encoding different DCLK isoforms. Likewise, the genomes of the two invertebrate species did not show any conserved region corresponding to DCLK sequences. This supports the hypothesis that the DCLK-encoding gene is absent in invertebrates.
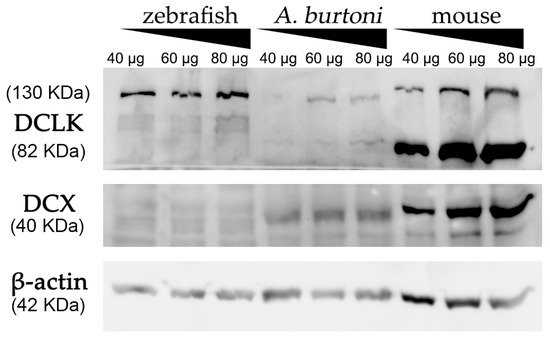
4. Western Blot Analysis Confirms the Presence of DCX in Burton’s Mouthbrooder Fish and Mice, but Not Zebrafish
Using a commercially available anti-DCX antibody, western blot assays revealed an intense and specific band of 40 KDa in protein samples from mice and
A. burtoni fish (
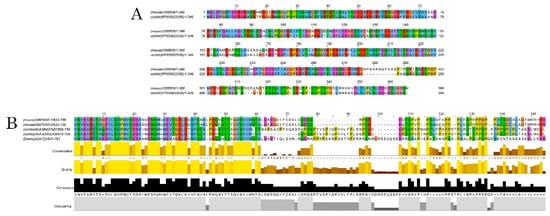
Figure 2 and Figure S3), which corresponded to the weight described for DCX protein (information related to sequence similarity between mice and
A. burtoni DCX can be found in
Figure 3A). No specific DCX band was found in zebrafish samples (
Figure 2). A positive immunoreactive band for β-actin (loading control) in all samples proved that protein lysates from zebrafish brain were correct. In contrast, DCLK expression was found in protein lysates from the three studied organisms (
Figure 2 and Figure S3); a strong immunoreactive band at 82 KDa is observed in lysates from mice brains, as well as a less intense band at 130 KDa. These two isoforms were also detected in samples from the
A. burtoni brain, especially when 60 mg and 80 mg proteins were loaded, although the expression of the 82 KDa isoform is significantly lower. In the case of zebrafish, a specific immunoreactive 130 KDa band was found for the three tested protein concentrations (information related to conservation of the immunogen sequence among the studied species can be found in
Figure 3B).
Figure 2. Western blot analysis of protein extracts derived from zebrafish, A. burtoni, and mice brains. Increasing concentrations of proteins were loaded in each well (40, 60 and 80 μg per well). Analysis shows the expression of DCX in A. burtoni and mice, but not in zebrafish extracts. β-actin was used as the loading control after the membrane stripping.
Figure 3. Alignments of DCX and DCLK amino acid sequences. Conserved residues between mice and A. burtoni DCX proteins (A); Conservation of the DCLK2 immunogen sequence use to detecting DCLK protein among the studied species (B).