Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ying Liang and Version 2 by Peter Tang.
Bioactive peptides are short peptides consisting of 2-20 amino acid residues. They have positive effects on body functions and generally have antibacterial, antihypertensive, antioxidant, and anti-inflammatory effects. Some exogenous bioactive peptides have been shown to have promising anti-aging effects. These exogenous peptides may have a mechanism similar to endogenous peptides, and some can even regulate the release of endogenous active peptides and play a synergistic role with endogenous active peptides.
- bioactive peptide
- anti-aging
- rodents
1. Introduction
In modern society, the extension of average life expectancy and the decreased birth rate have led to aging-related burdens across many regions [1][2][1,2]. Aging is a dynamic process associated with accumulated cell damage, a decline in biological function, and susceptibility to disease occurring over time [3]. A common and widely recognized mechanism for aging is oxidative damage caused by the accumulation of reactive oxygen species (ROS) [4], resulting from decreased antioxidant capacity, mitochondrial dysfunction, inflammation, etc. [5]. Aging can lead to multiple age-related diseases (ARDs) [6], such as cancer, Alzheimer’s disease (AD), cardiovascular disease (CVD), metabolic syndrome, obesity, fatty liver, and many other chronic diseases. The aging process inevitably involves the aging of cells, which is usually caused by damage at the molecular and cellular level by long-term exposure to endogenous and exogenous stressors. These damaged cells eventually lose their proliferative capacity and promote aging at an organism level [7]. These senescent cells can release a variety of pro-inflammatory factors and chemokines to promote cellular dysfunction, causing senescence-related diseases. In the process of skin aging, oxidative stress and inflammation can increase the activity of matrix metalloproteinases (MMPs) and increase the degradation of collagen, resulting in skin sagging and wrinkle formation. In some neurodegenerative diseases, such as AD, oxidative stress and inflammation can increase the accumulation of amyloid plaques (Aβ) and promote lesions in the brain. Oxidative stress and inflammation also play an important role in the aging of several other organs, such as the heart, liver, and kidneys. Collectively, these pathological changes can cause a variety of complications that affect multiple systems in the body. Thus, ARDs seriously impact the quality of life, shorten the lifespan, and bring a heavy burden to families and society. Therefore, in-depth studies of aging are particularly important.
Bioactive peptides are short peptides consisting of 2–20 amino acid residues. They have positive effects on body functions and generally have antibacterial, antihypertensive, antioxidant, and anti-inflammatory effects [8]. Natural bioactive peptides can be generally divided into two categories: endogenous peptides, which are naturally released from precursor proteins and secreted from cells, and exogenous peptides, which are produced by enzymatic hydrolysis of proteins or by biosynthesis or organic synthesis [9][10][9,10]. Bioactive peptide resources have been found in plants (soybeans, walnuts, rice bran, etc.), animals (some fish, dairy products, etc.), and some fungi and bacteria (yeast, lactic acid bacteria, etc.). The bioactive peptides used in early research were mainly derived from milk, cheese, and other dairy products. As research has progressed, active peptides have also been derived from other foods, including animal products as well as plant products [11]. They have been widely used in animal research, especially in rodents, but with limited research in humans. This is because rodents are easy to breed in the laboratory setting, have a short life cycle, and can be rapidly bred. Rodents also share similar genes and physiological functions with humans, making them ideal experimental animal models [12][13][12,13].
2. Bioactive Peptides Delay Skin Aging
2.1. Skin Aging
Skin is the largest organ and the body’s first barrier of defense against external pathogens. The skin protects the body from environmental damage and invasion of pathogens, and it is responsible for managing body temperature, sensation, and secretion function. Aging can cause different degrees of skin damage and interfere with the normal physiological function of other organs in the body [14][17]. The etiology of skin aging includes many factors. Among them, internal aging and photoaging are most common. Aging can alter the structure, function, and appearance of the skin, eventually leading to the increase of wrinkles, loss of elasticity, sagging, and pigment precipitation [15][18]. The main mechanisms of skin aging are the decrease of antioxidants in the skin, inflammation, and the degradation of collagen by increased MMPs [16][19]. Anti-aging bioactive peptides often act on these aging mechanisms. For example, oral collagen hydrolysates (CHs) can inhibit the activity of MMPs to reduce the degradation of collagen fibers [17][20]. Active peptides can reduce skin photoaging by scavenging free radicals [18][21]. Some bioactive peptides can reduce inflammation. In general, both endogenous and exogenous active peptides can down-regulate the factors causing skin aging. We discuss these in detail below.
2.2. Antioxidant Peptides in Delaying Skin Aging
Bioactive peptides derived from some animal proteins have antioxidant activity. These bioactive peptides can delay skin aging by regulating oxidative stress (Figure 1). For example, the collagen peptide extracted from the swim bladder of Sturgeon can increase the activities of catalase (CAT), SOD, and GSH peroxidase (GSH-PX) and decrease the activity of MMPs in skin tissue from Sprague-Dawley rats, as well as reduce the degradation of collagen by MMPs [19][22]. In recent years, some insect proteins with biological activity have also been found. For example, Eupolyphaga sinensis walker polypeptides (EPs) is a polypeptide mixture with a molecular weight of less than 3.3 kDa obtained from enzymatic digestion that can significantly improve the activity of antioxidant enzymes and reduce the generation of harmful free radicals. Thus, EPs can reduce the UV-irradiation-induced increase in epidermal thickness and elastic fiber breakage and restore the content of collagen [20][23]. In both cases, the mechanism of action of these exogenous active peptides is mainly to improve the activity of antioxidant enzymes in the skin and reduce the activity of MMPs and the degradation of collagen.
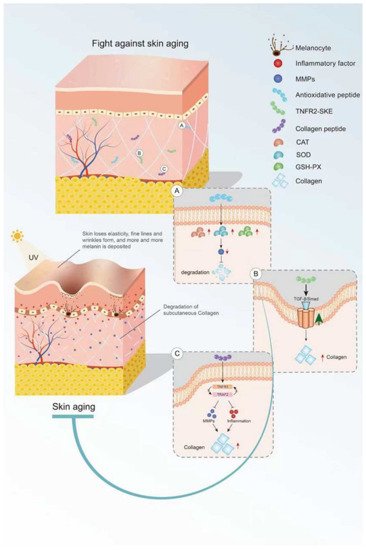

Figure 1. Mechanism of bioactive peptides in delaying skin aging. (A) Antioxidant peptides can increase the activity of antioxidant enzymes. (B) Bioactive peptides retard skin aging through the TGF-β/Smad pathway. (C) Active peptides inhibit inflammation and MMP activity. This figure cannot be reproduced without author permission.
2.3. Anti-Inflammatory Peptides in Delaying Skin Aging
Some endogenous peptides with anti-inflammatory effects have been used to delay skin aging. The tripeptide TNFR2-SKE (362.4 Da) derived from the tetrapeptide of TNF receptor-associated factor 2 (TNFR2) showed a good protective effect against skin photoaging. TNFR2-SKE can block the interaction between TNFR1 and TRAF2 and inhibit the inflammation induced by TNF-2 (Figure 1). Intraperitoneal administration of TNFR2-SKE to UVB-irradiated six-week-old male DBA/2 mice was shown to significantly improve epidermal thickness and pigment cell proliferation [21][24]. MOTS-C is a 16-peptide from the MDP family derived from mitochondria with a molecular weight of 2174.61 Da. This bioactive peptide can regulate cell metabolism and inflammation [22][23][25,26]. In a D galactose-induced aging mouse model, treatment with MOTS-c was shown to increase collagen fiber content in the dermis by increasing NRF2 and MFN2 and decreasing interleukin-6 (IL-6). The anti-aging activity of MOTS-c is likely achieved by reducing inflammation [24][27]. Thus, both TNFR2-SKE and MOTS-C active peptides showed good performance in significantly alleviating skin inflammation and increasing collagen fiber content in mice. These endogenous active peptides can delay skin aging through their anti-inflammatory effects. However, there are many endogenous anti-inflammatory polypeptides in the body, and their anti-aging effects on the skin remain to be explored.
2.4. Peptides in Reducing Collagen Hydrolysis
Collagen is the main component of the dermis, and its content decreases with age. Skin sagging and wrinkles are caused by a decrease in collagen content. It is noteworthy that oral CHs can reduce skin laxity and wrinkles [25][28] and delay skin aging. Fish skin and fish scales are generally rich in collagen. Two collagen hydrolysates (ACH and CCH) prepared from fish skin can up-regulate the transforming growth factor β (TGF-β)/Smad signaling pathway related to collagen synthesis and increase the amount of collagen. CHs have a good protective effect on skin laxity, as shown in 13-month-old female KM mice [26][29]. Collagen hydrolysate CPNS (Gly-Pro and Pro-Hyp) [27][30] and CP [28][31] prepared from fish scales can significantly attenuate the increase in epidermal thickness and water loss and the decrease in dermal hyaluronic acid (HA) induced by UVB irradiation, as well as recover HA loss by regulating hyaluronan synthases 1 (HAS1), hyaluronan synthases 2 (HAS2), and hyaluronidase 2 (HYAL2). Another elastin hydrolysate (EH) prepared from the bovine artery is composed of four polypeptides: Gly-Leu-Pro-Tyr (GLPY), Pro-Tyr (PY), Gly-Leu-Gly-Pro-Gly-Val-Gly (GLGPGVG), and Gly-Pro-Gly-Gly-Val-Gly-Ala- Leu (GPGGVGAL). EH can inhibit UV-induced skin thickening and sebaceous gland hyperplasia in mice and promote moisturizing of the skin. GLPY and GPGGVGAL have better inhibitory effects on elastase and thus can reduce extracellular matrix (ECM) degradation and improve the activity of UV damaged fibroblasts [29][32]. Collagen hydrolysis is the main cause of skin sagging, and the supplement of some collagen hydrolytic peptides can reduce the hydrolysis of collagen by MMPs. However, the detailed underlying mechanism is still unclear and needs to be further explored.
3. Bioactive Peptides and Brain Aging
3.1. Brain Aging
In the process of aging, brain function will gradually decline, which is manifested by a decline of learning ability and memory, as well as attention, decision-making ability, sensory perception, and motor ability. The prevalence of some neurodegenerative diseases, such as AD, Parkinson’s disease (PD), and stroke, also increases with age. The development of these diseases is related to mitochondrial dysfunction, accumulation of oxidative damage, and increased inflammation [30][33]. AD is the most common neurodegenerative disease. Currently, abnormal folding of Aβ1-42 produced by the metabolism of amyloid precursor protein (APP) is considered to be the main cause of AD pathology [31][34]. Iron is involved in many biological processes in the brain and plays an important role in maintaining normal brain function. However, an iron imbalance can cause toxic effects on the brain. When the iron concentration is too high, it can increase the misfolding of Aβ and promote the development of AD [32][35]. The role of oxidative stress and inflammation in the development of AD is well known, and some new therapeutic targets have become research hotspots. Serotonin receptors (5-HT4R) have been found to reduce Aβ production. Many 5-HT4R agonists have been studied, but their potential therapeutic effect on AD has rarely been studied in vivo [33][36]. Glycosylation of proteins produces advanced glycation end products (AGEs) that can cause neurodegeneration. When glyoxalase activity is reduced, the ability of these toxic glycosylated proteins to be eliminated is significantly reduced, leading to neurological disease [34][37]. The relationship between the gut microbiome and aging and the development of AD has been confirmed, but no clear mechanism has been elucidated. In a recent report, thwe researchers found that intestinal dysregulation of Firmicutes and Bacteroidetes promotes T helper 1 (Th1) cell infiltration and promotes microglia differentiation in a pro-inflammatory direction. This may be related to the development of AD [35][38]. Bioactive peptides can exert their anti-aging effect on the brain through various mechanisms. They can increase antioxidant enzyme activity, reduce inflammation, increase the removal ability of iron and AGEs, increase expression of 5-HT receptors, and regulate the gut microbiota.
3.2. Antioxidant Peptides in Delaying Brain Aging
Carnosine (CAR) is an endogenous dipeptide (β-Ala-L-His) existing in muscle, blood, and the brain. CAR has good antioxidant activity and can attenuate neurological diseases caused by aging; CAR supplementation reduces the accumulation of Aβ in the hypothalamus and prefrontal cortex of aging rats and has potential therapeutic effects on AD [36][39]. After CAR treatment, GSH levels and SOD and GSH-Px activity were increased, whereas acetylcholinesterase (AChE) activity was significantly decreased (Figure 2), and there was a significant reduction in neuronal apoptosis, brain edema, and inflammation in D-galactose treated rats [37][40]. With aging, iron gradually accumulates and induces the generation of free radicals, promoting the formation of Tau and Aβ oligomers, which are neurotoxic and the main cause of AD [38][41]. The amount of iron found in the brains of AD patients is much higher than that of normal brains, suggesting that excess iron may be one of the causes of AD [32][39][35,42]. To better understand the effects of iron, researchers have synthesized the peptides with the ability to remove iron ions. Pentapeptide YHEDA (Tyr-His-Glu-Asp-Ala) and polypeptide mixture HAYED (5) Five (His-Ala-Tyr-Glu-Asp) repeat sequences are two synthetic active peptides with good iron ion scavenging ability (Figure 2). They can prevent the decrease of blood oxygen metabolism, inhibit the generation of free radicals, and reduce the damage in brain tissue, effectively improving cognitive impairment in senescent (SN) mice (25 months old) [40][41][43,44]. However, many high-quality natural antioxidant peptides have yet to be discovered and utilized in anti-aging studies. For example, many plant-derived bioactive peptides have antioxidant activities, and the research and development of these active peptides in aging studies will be of great significance in delaying brain aging [42][45].
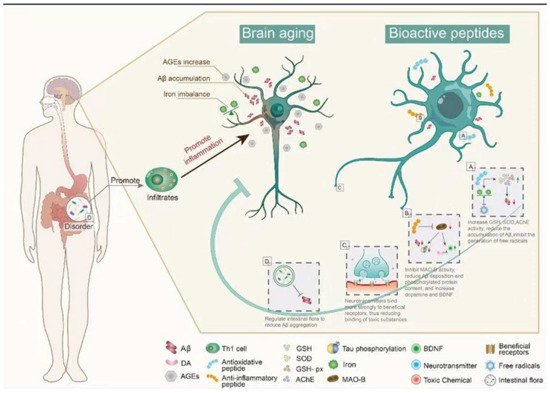

Figure 2. The main mechanism of bioactive peptides in delaying brain aging. (A) Bioactive peptides reduce Aβ accumulation by regulating oxidative stress. (B) The bioactive peptides inhibit the activity of MAO-B, up-regulate BDNF, and reduce the aggregation of Aβ. (C) Bioactive peptides reduce brain damage caused by toxic substances in the brain. (D) Bioactive peptides reduce Aβ aggregation by regulating intestinal microbiota. This figure cannot be reproduced without author permission.
3.3. Anti-Inflammatory Peptide in Delaying Brain Aging
Synthetic bioactive peptides are being increasingly produced for the treatment of different diseases. Liraglutide, a synthetic long-acting glucagon-like peptide 1 (GLP-1) analog, is widely used in the treatment of diabetes mellitus and CVDs. Recently, it has been speculated that liraglutide may have neuroprotective effects [43][44][46,47]. In senescence accelerated mouse P8 (SAMP8) mice (model of AD-like dementia), liraglutide treatment can improve spatial long-term memory and increase the number of hippocampal neurons [45][46][48,49]. The active peptides in dairy products have been long known, and there have been some reports that these active peptides can delay brain aging, mainly with the improvement of AD symptoms. The Whey protein hydrolysate tryptophan-methionine and tryptophan-tyrosine, extracted from fermented dairy products, can improve the cognitive impairment of AD mice. Inflammation and Aβ1-42 deposition in the cerebral cortex and hippocampus are also significantly reduced in 5 × FAD transgenic mice fed tryptophan-tyrosine [47][50]. Notably, Tryptophan-Tyrosine dipeptide and whey protein hydrolysate GTWY (Gly-Thr-Trp-Tyr) can increase dopamine (DA) content in the hippocampus and frontal cortex of AD mice by inhibiting the activity of monoamine oxidase B (MAO-B) [48][49][50][51,52,53]. β-lactolin, an active polypeptide extracted from whey protein hydrolysate, has been shown to improve cognitive impairment. Specifically, β-lactolin can reduce amyloid plaque deposition and phosphorylated Tau protein content in the cerebral cortex of 5 × FAD transgenic mice (AD mice), as well as increase DA and BDNF levels, thereby improving the cognitive impairment [51][54]. BDNF is one of the most widely distributed neurotrophic factors in the brain, and it plays an important role in regulating synaptic growth, neuroprotection, and affecting memory and cognition in vivo [52][55]. β-lactolin can increase the expression of BDNF in vivo (Figure 2). This is an example of how exogenous active peptides have a regulatory effect on endogenous active substances. Thus, exogenous active peptides not only play a therapeutic role in some antioxidant and anti-inflammatory pathways but also enhance the expression of endogenous active peptides to treat some diseases. The mechanism of action of exogenous active peptides may differ from endogenous ones, but they can supplement the body’s defense system.
3.4. Regulation of Peptide Receptors in Delaying Brain Aging
Serotonin (5-HT) is an important neurotransmitter that is involved in a variety of brain activities and functions. 5-HT receptors decrease gradually in the aging process. Serotonergic neurons are widely distributed in the brain. Reduction of 5-HT receptors can cause functional impairment of these neurons and lead to cognitive impairment. CAR is a dipeptide extracted from the meat. It can enhance 5-HT binding to its receptor and restore the regional senage-induced decrease in serotonin to normal levels [53][54][56,57]. Pituitary adenylate cyclase activated polypeptide (PACAP) is an endogenous active polypeptide with 38 amino acid residues and has a neuroprotective effect. It is widely distributed in the brain, pancreas, gonad, and respiratory tract. PACAP38 can be cleaved to form a 27 amino acid polypeptide, PACAP27 [55][58]. The level of PACAP gradually decreases in the normal aging process, and decreased PACAP levels have been found in the brain tissues of AD patients [56][59]. PACAP27 and PACAP38 can reduce the accumulation of Aβ in the brain by activating pituitary adenylate cyclase-activating polypeptide (PAC1), which causes the shedding of the receptor for advanced glycation end products (RAGE) of late glycation end products on the cell surface [57][60]. In summary, these peptides act on receptors, promoting the binding of beneficial receptors in neurons but reducing the binding of toxic substances.
3.5. Intestinal Microbiota Regulation by Peptides in Delaying Brain Aging
The link between the gut microbiota and AD is widely recognized, and many substances, including bioactive peptides, have been reported to regulate the gut microbiota. Some active peptides can regulate the intestinal microbiota in a beneficial direction by reducing Aβ aggregation, which has a potential role in the treatment of AD by regulating the intestinal microbiota [58][61]. The walnut protein hydrolysate PW5 (Pro-Pro-Lys-Asn-Trp) identified from walnut protein can reduce Aβ aggregation and improve cognitive impairment in mice by regulating intestinal microbiota (Figure 2). PW5 fed to APP/PS mice (AD mice) can increase firmicutes in the intestinal microbiota, which may be associated with reduced Aβ aggregation in mice [59][62]. The association between intestinal microbiota and AD has been widely recognized, and many bioactive peptides have been used to regulate intestinal microbiota to improve AD symptoms, but the mechanism is still not deeply studied, and further exploration is needed.
4. Bioactive Peptides and Aging in Other Organs
Aging is an irreversible biological process. Organs in the body cannot avoid aging. This leads to a variety of chronic diseases, including CVD, chronic obstructive pulmonary disease (COPD), intermittent lung disease, and asthma [60][61][63,64]. The aging processes of these important organs are correlated, and complications of one organ often lead to multi-organ disease. For example, lung aging causes COPD, which causes systemic inflammation and increases the risk of non-alcoholic liver disease. Moreover, people with non-alcoholic liver disease are more likely to have chronic kidney disease (CKD) and CVD. Oxidative stress and inflammation play an important role in the pathogenesis of these diseases.
4.1. Lung Aging
COPD is a major form of lung disease characterized by chronic inflammation of the windpipe. Aging and smoking are the main causes of COPD. People over the age of 65 are five times more likely to develop the disease than younger people [62][63][65,66]. COPD is often associated with metabolic abnormalities, CVD, skeletal muscle atrophy, and other chronic diseases. In the later stages of COPD, arteriosclerosis, oxidative stress, and inflammation are the main mechanisms of its progression. Persistent inflammation disrupts the normal function of the lungs and is one of the causes of other complications [64][67]. Other researchers point to systemic inflammation from COPD as a major cause of non-alcoholic fatty liver disease (NAFLD) [65][68].
