Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Marina Stogniy and Version 2 by Rita Xu.
Recent decades have demonstrated a growing interest in the chemistry of 7,8-dicarba-
nido
-undecaborante anion (
nido
-carborane) due to the wide possibilities of its application from medicine to catalysis. One of the main approaches to the modification of
nido
-carborane cluster is the ring-opening reactions of its cyclic oxonium derivatives with various nucleophiles, which opens practically unlimited prospects for the incorporation of
nido-carborane into various macro- and biomolecules.
- nido-carborane
- half-sandwich complexes
- oxonium derivatives
- ring-opening reactions
1. Introduction
This anionic boron cluster with an open pentagonal face was synthesized for the first time by M. F. Hawthorne more than 50 years ago by deboronation of 1,2-dicarba-closo-dodecaborane (ortho-carborane) with potassium hydroxide in methanol [1][2][2,3]. Later various milder deboronating agents including amines [3][4][5][6][4,5,6,7] and fluoride ion [7][8][9][10][8,9,10,11] were proposed. Deprotonation of nido-carborane with strong bases results in the dicarbollide dianion [7,8-C2B9H11]2−, which was recognized as an isolobal analogue of the cyclopentadienyl anion [11][12]. Due to this, the dicarbollide dianion is considered as an unusual three-dimensional π-ligand for the synthesis of metal complexes [12][13][14][13,14,15], which can be used as catalysts in a variety of chemical processes [15][16][16,17]. Another important area of use for nido-carborane derivatives as water-solubilizing boron moieties is the design of potential drugs for boron neutron capture therapy of cancer [17][18][19][20][21][22][18,19,20,21,22,23]. In addition, based on nido-carborane derivatives, it is possible to obtain reagents for radioimaging of tumors [23][24][25][26][27][24,25,26,27,28], new optical and luminescent materials [28][29][30][31][32][33][34][35][36][29,30,31,32,33,34,35,36,37], ionic liquids [37][38][38,39], etc. Thus, the development of convenient approaches to the modification of the nido-carborane cluster remains highly relevant.
Currently the main approach to the synthesis of nido-carborane derivatives is based on the deboronation of the corresponding ortho-carborane derivatives. This approach is widely used for the synthesis of C-substituted derivatives of nido-carborane, as well as derivatives containing substituents at the lower belt of the nido-carborane cage [39][40]. There are several general methods for the synthesis of B-substituted derivatives with substituents in the upper belt of the nido-carborane cage; however, most of them are used to obtain asymmetrically substituted derivatives [9-X-7,8-C2B9H11]− [40][41][42][43][44][45][46][47][48][41,42,43,44,45,46,47,48,49].
Several approaches to the synthesis of symmetrically substituted derivatives of nido-carborane [10-X-7,8-C2B9H11]− with boron–sulfur [49][50][50,51] and boron–nitrogen [51][52][53][54][52,53,54,55] bonds have also been developed recently; however, the greatest interest is the functionalization of nido-carborane via its cyclic oxonium derivatives. Previously, this approach was successfully used for the synthesis of numerous derivatives of closo-decaborate and closo-dodecaborate anions [55][56][56,57], as well as sandwich bis(dicarbollide) transition metal complexes [57][58].
2. Synthesis of Oxonium Derivatives of nido-Carborane
To date, oxonium derivatives of nido-carborane have been synthesized with all cyclic ethers used as common solvents, including tetrahydrofuran, 1,4-dioxane, and tetrahydropyran. The first oxonium derivative was obtained as early as 1969 by the reaction of the potassium salt of nido-carborane with FeCl3 in a mixture of benzene and tetrahydrofuran. The reaction gave a mixture of asymmetric 9-(CH2)4O-7,8-C2B9H11 and symmetric 10-(CH2)4O-7,8-C2B9H11 which were separated by column chromatography in benzene. Similar mixture of isomers was obtained for the C,C-dimethyl derivative of nido-carborane as well [58][59] (Scheme 1).
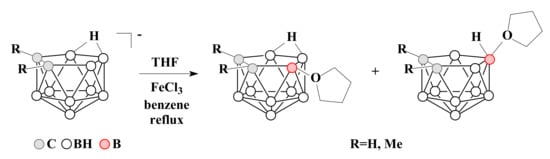

Scheme 1. The synthesis of 9-(CH2)4O-7,8-C2B9H11 and 10-(CH2)4O-7,8-C2B9H11 by the reaction of nido-carborane with FeCl3 in a THF–benzene mixture.
The proposed mechanism of this reaction includes the initial abstraction of hydrogen hydride from position 9 by iron(III) chloride as a Lewis acid with the formation of a quasi-borinium cation. In the absence of strong nucleophiles, this intermediate can be isomerized to a more stable symmetric form with a quasi-electrophilic center at position 10, which is then attacked by an ether solvent molecule as a weak but most accessible nucleophile. As a result, a mixture of 9- and 10-substituted isomers is formed [59][60]. This mechanism is known as electrophile-induced nucleophilic substitution (EINS) and is considered as one of the main mechanisms of substitution of hydrogen atoms in polyhedral boron hydrides.
Later, it was found that the reaction of nido-carborane with HgCl2 in tetrahydrofuran leads to the selective formation of the symmetrically substituted product 10-(CH2)4O-7,8-C2B9H11 [59][60][60,61]. In a similar way, the derivatives with 1,4-dioxane 10-O(CH2CH2)2O-7,8-C2B9H11 [59][60] and tetrahydropyran 10-(CH2)5O-7,8-C2B9H11 [61][62][62,63] were synthesized.
It is assumed that the selectivity of the reactions in these cases is determined by the fact that the endo-polyhedral hydrogen atom of nido-carborane cage is replaced at the first stage of the reaction by a mercury atom with the formation of η1-metallacarborane, in which mercury is bound to the B(10) atom of the dicarbollide ligand [63][64][65][66][64,65,66,67]. Heating of this complex results in elimination of mercury and generation of quasi-electrophilic center at position 10 followed by its attack by the nucleophile [59][60].
Another convenient method for the synthesis of 1,4-dioxane derivative of nido-carborane is to heat in 1,4-dioxane the protonated form of nido-carborane 7,8-C2B9H13, which is formed by treating a suspension of the triethylammonium salt of nido-carborane with concentrated sulfuric acid in toluene [67][68].
The symmetrically substituted tetrahydrofuran derivative 10-(CH2)4O-7,8-C2B9H11 can be obtained by the reaction of tetramethylammonium salt of nido-carborane with AlCl3 in a mixture of tetrahydrofuran and acetone [68][69]. The symmetrically substituted tetrahydrofuran and 1,4-dioxane derivatives of nido-carborane were also synthesized by the reactions of nido-carborane with the corresponding ethers in the presence of acetaldehyde or formaldehyde and hydrochloric acid in a mixture of water and toluene [69][70]. The methods of synthesis of symmetrically substituted oxonium derivatives of nido-carborane are summarized in Scheme 2.
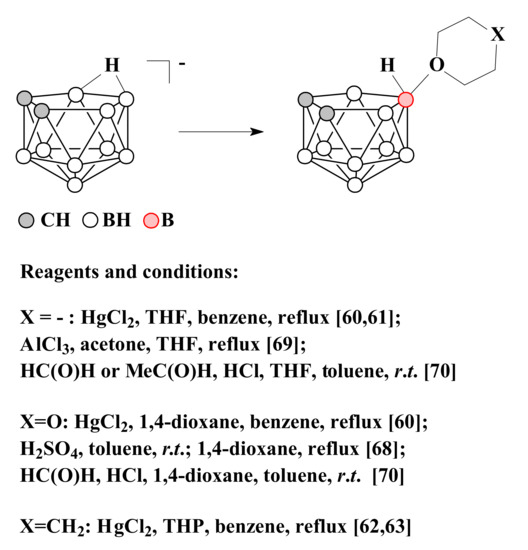

Scheme 2. The different synthetic pathways to the 10-(CH2)4O-7,8-C2B9H11, 10-O(CH2CH2)2O-7,8-C2B9H11 and 10-(CH2)5O-7,8-C2B9H11.
The molecular structure of the 1,4-dioxane derivative of nido-carborane was determined by single crystal X-ray diffraction [70][71] (Figure 1).
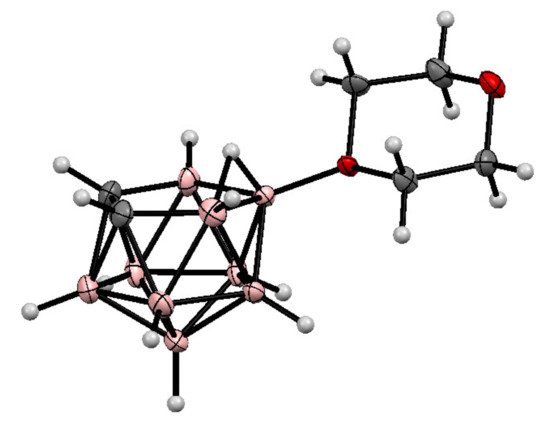

Figure 1. The molecular structure of 10-O(CH2CH2)2O-7,8-C2B9H11.
It should be noted that, in addition to cyclic oxonium derivatives of nido-carborane, some acyclic oxonium derivatives such as the dimethyloxonium 9-Me2O-7,8-C2B9H11 and 10-Me2O-7,8-C2B9H11 [71][72] and the diethyloxonium 9-Et2O-7,8-C2B9H11 [72][73] and 10-Et2O-7,8-C2B9H11 [69][72][73][70,73,74] derivatives are known as well. The molecular structure of the 10-diethyloxonium derivative of nido-carborane was determined by single crystal X-ray diffraction [72][73] (Figure 2). The formation of the 1,2-dimethoxyethane derivatives 9-MeOCH2CH2(Me)O-7,8-C2B9H11 and 10-MeOCH2CH2(Me)O-7,8-C2B9H11 was also reported, however they have low stability and easily lose the methyl group, giving the corresponding alkoxy derivatives [74][75].
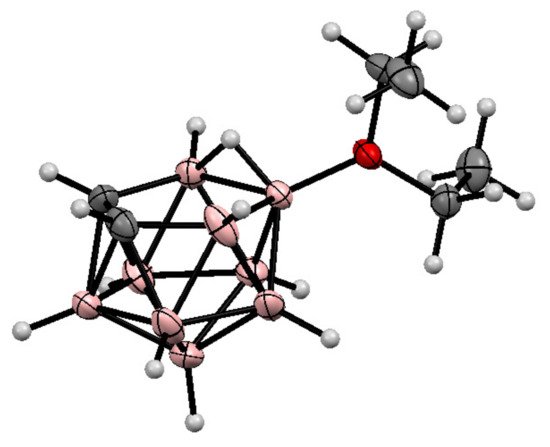

Figure 2. The molecular structure of 10-Et2O-7,8-C2B9H11.
