Cutaneous Melanoma (CM) is an aggressive and invasive cancer of the skin. Epigenetic mechanisms are fundamentally important for cancer initiation and development. The application of some insights may contribute to further progress in the diagnosis and therapy of melanoma, a deadly type of cancer.
- melanoma
- DNA methylation
- epigenetics
1. Etiology of Cutaneous Melanoma
2. Histone Modifications and Significance for Melanoma Progression
Multiple histone modification changes have been reported in CM, and some of them have been linked to tumor behavior. First, an increase in the global levels of demethylated histone H3 at lysine 9 (H3K9me2) has been found in melanoma samples, compared with the normal peritumoral skin tissue [6][15]. H3K9me2 recruits the heterochromatin protein 1 (HP1) which directs DNA methyltransferase 1 (DNMT1)-dependent DNA methylation in vivo [7][16] and plays a key role in the formation of transcriptionally inactive heterochromatin [8][17]. This histone modification is established by the histone methyltransferase G9a [7][16], which has been found significantly upregulated in melanoma patients [9][18] who have a poorer outcome [10][19]. Conversely, G9a silencing elevates the self-renewal capability of differentiated melanoma cells in a Sox2-dependent manner [11][20]. Sox2 is a master regulator of pluripotency in embryonic stem cells. In addition, inhibition of G9a induced cell death in diverse melanoma cell types and diminished tumor growth in vivo [12][21]. The importance of chromatin-modifying enzymes in regulating tumorigenesis was underscored in a zebrafish melanoma model. Overexpression of either SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) or suppressor of variegation 3–9 homolog 1 (SUV39H1), both enzymes methylating histone H3 on lysine 9 (H3K9), significantly accelerates melanoma formation [13][22]. Independently, it was shown that the H3K9-specific methyltransferase SUV39H1 establishes trimethylation at H3K9 at the promoter of the tumor suppressor gene retinoblastoma 1 (RB1), and this recruits DNA methyltransferase 3A, which mediates DNA methylation of the promoter. Thus, RB1 expression becomes epigenetically repressed, and, in turn, E2F1, which is inhibited by RB1, becomes transcriptionally activated. The authors show that this promotes UV-induced skin tumorigenesis in vivo. Conversely, depletion of SUV39H1 in melanoma cells leads to RB1 activation and reduced E2F1 transcriptional activity, inhibiting melanoma development [14][23]. The RB protein exerts tumor suppressor function by negative control of the cell cycle and by binding to E2F family members and repressing their functions at the promoters of genes, which are important for S-phase progression and cell proliferation [15][24]. RB interacts with histone acetyltransferases (HATs) and -deacetylases (HDACs), supports repair of DNA double-strand breaks (DSB), chromosome condensation, and silencing of repetitive sequences. Through its chromatin regulatory functions, it affects genomic stability [15][24]. E2F1 promotes cell proliferation but only develops its oncogenic properties when pathways that mediate E2F1-induced apoptosis are disabled [16][25]. A variety of functional studies show that melanoma cells reprogram their survival pathways and expand their intrinsic resistance to apoptosis during melanoma progression [17][26]. Interestingly, the p16INK4A–Rb–E2F pathway, which is an important regulator of cell cycle and differentiation, and its dysfunction can lead to oncogenesis, is altered in more than 80% of human neoplasias [16][25]. p16INK4A arrests the cell cycle in G1 by inhibiting CDK4 and CKD6, thereby preventing the inactivation of pRB [18][27]. In 72 melanomas greater than 1.0 mm and 29 metastases, its expression has been found lost in 100% of the cases [19][28]. Straume et al. reported the loss of p16 protein expression by promoter hypermethylation in 19% of primary cutaneous melanomas and in 33% of metastases [20][29]. The RB1-target E2F1 positively regulates the enhancer of zeste homolog 2 (EZH2), which is a histone–lysine N-methyltransferase enzyme and a core subunit of the polycomb repressive complex PRC2, which is responsible for global changes of chromatin architecture and essential in early development but is downregulated in normal adult tissues. EZH2 is an important driver of melanoma progression [21][30], and its increased activity leads to increased global H3K27me3. Increased H3K27me3 is an indicator of poor prognosis and is associated with aggressive and metastatic forms of melanoma [22][31]. The 5-year survival rate of EZH2-high patients was 48%, compared with 71% in the EZH2-low group [23][32]. The EZH2-mediated elevation of H3K27me3 has been described to be involved in epigenetic silencing of the tumor suppressor genes RUNX3 and CDH1 in advanced-stage human melanoma tissues [24][33].3. DNA Methylation Alterations in Melanoma
A study of 16 melanoma cell lines, an elevated methylation status was reported for the following gene promoters: ESR1 (50%), MGMT (50%), RARB2 (44%), RIL (88%), RASSF1A (69%), PAX7 (31%), PGRB (56%), PAX2 (38%), NKX2-3 (63%), OLIG2 (63%), HAND1(63%), ECAD (88%), CDH13 (44%), and CDKN2A/p16 (6%) [25][38]. Conversely, the genes CD2, EMR3, CARD15, EV12A, HLA-DP1, IFNG, IL2, ITK, KLK10, LAT, MPO, PSCA, PTHLH, PTHR1, RUNX3, and TNFSF8 have been found hypomethylated in 25 primary melanomas, compared with 29 benign nevi [26][39]. Interestingly, distinct hypermethylated genes have been found associated with genetic mutation subgroups, e.g., NF1 hypermethylation with NF1- and RAS-mutated melanomas, PTEN hypermethylation with BRAF-mutated, and CDK2A/B hypermethylation with BRAF-, RAS-, NF1-mutated and triple-WT melanomas [27][40]. Repetitive LINE-1 retrotransposon hypomethylation may result in their reactivation, LINE-1 RNA, and protein expression, and has been linked to apoptosis, DNA damage and repair, tumor progression, cellular plasticity, and stress response [28][41]. In 75% of 16 melanoma cell lines, significant hypomethylation of LINE-1 sequences was found [29][42].4. One-Carbon Metabolism in the Etiology of Epigenomic Aberrations in Melanoma
While a plethora of DNA methylation alterations occur in melanoma, it remains elusive how they are caused. It has been proposed that either active processes, e.g., an aberrant activity or function of DNMT enzymes, or passive ones, for instance, changes in epigenetic modifications that regulate targeting of DNA methylation, may be involved [30][36].
A major risk factor for bladder cancer is persistent exposure to the harmful carcinogens of tobacco smoking, which is estimated to account for 50% of tumors [31][44] and contributes to the high mutational rate in that cancer. There is thus a parallel to the etiology of melanoma, where exposure to another exogenous carcinogen—harmful UVB radiation—is the major cause. The previously proposed PrimeEpiHit hypothesis for UC [31][32][44,45] may, therefore, explain methylation alterations in CM carcinogenesis. According to this modified hypothesis, chronic UVB radiation exposure may occasionally also hit genes with key functions in one-carbon-group metabolism. As a result, their transcription may become impaired, and subsequently, their epigenetic status may be altered. Epigenetic silencing because of gene disruption has been experimentally demonstrated [33][46]. Interestingly, analyzing a comprehensive mortality rate dataset for 30 types of cancer for 52 provinces in Spain, spanning 1978–1992, it has been found that melanoma correlated with bladder and lung cancer, suggesting common risk factors and mechanisms [34][47]. Key genes involved in one-carbon-group metabolism of course comprise a very small percentage of the whole genome, but due to the chronic carcinogen exposure, nonetheless, this may occur at some minor frequency, which appears in accordance with the low incidence.
Impairment of key genes of one-carbon-group metabolism causes imbalances in the involved methyl group metabolic pathways. This disturbs the delicate SAM:SAH ratio and, consequently, genome-wide DNA methylation alterations, including LINE-1 hypomethylation that contributes to genetic instabilities; thus, cellular transformation occurs. Notably, this process could be enhanced by the well-described deficiencies of one-carbon-group metabolism associated with aging, which are likewise characterized by accumulation of SAH and DNA hypomethylation [35][48]. Age is an important risk factor for UC, as well as CM. Figure 1 provides an overview illustration of the key metabolites and enzymes involved in methyl group and polyamine metabolism and their interactions.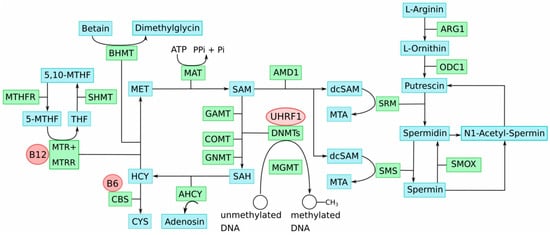
5. Epigenetic Diagnosis Based on Differential Chromatin Organization and DNA Methylation in Melanoma
Focusing first on cancer-specific differential chromatin organization, for taking advantage of it in cancer diagnosis and therapy, it suggests screening after sites of chromatin with distinct differential organized structures for various melanoma stages, e.g., the first mesenchymal stage, which arises after the epithelial–mesenchymal transition. Once detecting such promising chromatin areas with, e.g., a more relaxed chromatin organization in a pathogenic melanoma cell population, it would be able to target it either for diagnosis or even for therapy. For diagnosis, e.g., taking advantage of the limited effectiveness of micrococcus nuclease (MN) on closed chromatin, it would be possible to pretreat melanoma and reference DNA and provide cancer-cell-originated DNA templates of MN-affected integrity for assaying them by sensitive PCR assays, as previously demonstrated [39][55]. For therapy, those cancer-cell-specific, differential organized chromatin areas could be targeted by CRISPR/Cas in vivo, taking advantage of its selective preference feature. Evolved as an adaptive immunity system that protects bacteria and archaea against phages and plasmids [40][56] by directing sequence-specific Cas9 endonuclease-mediated double-strand DNA cleavage (DSB) to the intruder’s DNA, and hence destroying its genetic information [41][57], this system has evolutionarily never been encountered as a substrate DNA, which is organized in complex, high-order, structured chromatin and, hence, has a high preference for binding to more easily accessible chromatin regions [42][43][44][58,59,60].
A further basic approach is to find within a certain distinct cancer cell population of interest, differentially methylated CpG dinucleotides forming a distinct, consistent, and characteristic methylation signature for this cell population. In the former investigations on prostate cancer and urothelial cancer specimens from patients, it was able to discover such unique DNA methylation signatures. The proposed strategy [32][45] is to screen using DNA methylation array technology after such differentially methylated CpG regions, and once detected, to validate and precisely dissect the cancer cell-specific DNA methylation pattern by bisulfite genomic sequencing [45][67]. Afterward, based on this information, it can define the outmost most suitable and robust MSPCR primers to sensitively detect this methylation signature.
Furthermore, a new methodological approach to separate cell-free DNA from cellular DNA and unreservedly apply bisulfite genomic sequencing and MSPCR on this pure cell-free DNA is recently presented. It is established that all tumors shed their cell-free DNAs that bear their unique DNA methylation patterns into the bloodstream [45][67], hence providing an easily accessible and exciting Achilles’ heel of cancer for effective diagnosis. For instance, PTEN promoter methylation was reported in cell-free DNA from 62% of the melanoma serum samples examined by pyrosequencing, indicating a good correlation with the same epigenetic alteration found in paired melanoma tissues [46][69].
MSPCR has been improved and enables, for the first time, relative quantification of DNA methylation in samples with identical and unequal genetic settings [47][71]. This is of importance for cancer samples, which often yield genetic aberrations, including melanoma, characterized by a higher number of chromosomal structural aberrations [48][72], and bladder carcinoma, characterized by early (pTa, pT1) chromosomal changes and imbalances [49][73]. A conventional normalization of MSPCR by using a housekeeping gene such as GAPDH or the ACTB gene may result in false results, especially if tumor samples from different patients must be compared, because, e.g., the copy number of the DNA segment containing the housekeeping gene chosen for normalization may vary in cancer DNA. The reliable measurement of DNA methylation by using MSPCR is based on DNA amplification, and it is extremely hindered by a variable number of template segments. In contrast, by using the idiolocal normalization of real-time, methylation-specific PCR (IDLN–MSP) [47][71], the normalization loci are chosen adjacent to the targeted loci. With this approach, it is guaranteed that in real-time MSPCR, the number of normalization loci is always as high as the number of targeted loci, reducing false-negative results in a significant manner, and this contributes to a significant improvement of DNA methylation measurements by MSPCR in tumor samples of different patient origin [47][71].
