Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disorder, affecting 1 out of 500 adults globally. It is a widely heterogeneous disorder characterized by a range of phenotypic expressions, and is most often identified by non-invasive imaging that includes echocardiography and cardiovascular magnetic resonance imaging (CMR). Within the last two decades, cardiac magnetic resonance imaging (MRI) has emerged as the defining tool for the characterization and prognostication of cardiomyopathies. With a higher image quality, spatial resolution, and the identification of morphological variants of HCM, CMR has become the gold standard imaging modality in the assessment of HCM. Moreover, it has been crucial in its management, as well as adding prognostic information that clinical history nor other imaging modalities may not provide.
- cardiac MRI
- hypertrophic cardiomyopathy
- sudden cardiac death
1. Introduction
2. Cardiovascular Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy Evaluation
2.1. Definition of HCM
2.2. Cardiovascular Magnetic Resonance vs. Echocardiography
Whilst conventional echocardiography is considered the first line imaging modality for the clinical diagnosis of HCM, it is operator dependent and often limited by its acoustic windows and lack of tissue characterization [9][11]. Conversely, CMR allows a more comprehensive and detailed evaluation of hypertrophy, with accurate measurements of wall thickness, chamber size, and distribution of hypertrophy (Table 1). It is also capable of evaluating coronary flow reserve, contractility, and tissue perfusion, permitting the reproducible assessment of cardiac abnormalities [10][12]. One of the significant advantages of CMR includes detailed anatomical assessment in different planes, providing a three-dimensional representation of anatomy [11][13]. Thus, contouring the epicardial and endocardial borders allows the calculation of quantitative parameters, including consistent measurements of atrial and ventricular function and volumes. A typical CMR examination involves electrocardiographic (ECG) synchronized cine acquisitions, at a strength of 1.5 or 3T [12][14]. There are three main techniques used in clinical CMR, which include spin echo imaging, gradient echo imaging, and flow velocity encoding [11][13]. Steady-state free precession (SSFP), related to gradient echo imaging, involves generating high temporal and spatial resolution cine images, which are important for functional assessment [13][15].| Advantages of Cardiovascular Magnetic Resonance Imaging |
|---|
| Identification of HCM phenotypes |
| Accurate quantification of maximal wall thickness |
| Assessment of co-existing valvulopathies |
| Volume analysis and quantification |
| Perfusion and strain analysis |
| Assessment of LVOT and cause |
| Offers differential diagnosis |
| Risk stratification through identification of fibrosis |
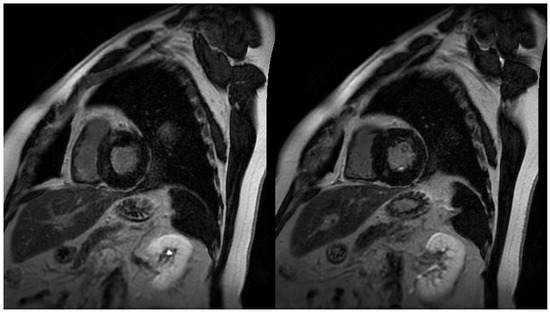
2.3. Late Gadolinium Enhancement
The identification and quantification of LGE is a valuable feature of CMR, changing the paradigm in how ischemic and non-ischemic myocardial diseases are assessed (Figure 1). It represents myocardial fibrosis and is a predictor of both a higher mortality rate and the progression towards heart failure amongst HCM patients [14][19]. The mean reported prevalence of LGE is 65%, but may be present in up to 86% of HCM patients [7][15][7,20]. Typical patterns include localization to the mid-wall, located in segments with the greatest LVH, and at RV insertion points. Thus, peculiar patterns of LGE, during the assessment of LVH, can be attributed to other diagnoses that may include, but are not limited to, Anderson–Fabry disease or cardiac amyloidosis (CA). The distinction between the location of LGE includes the type of fibrosis, whereby intramural mid-wall LGE is considered a marker for replacement fibrosis, whilst LGE at RV insertion points suggests interstitial fibrosis [16][21]. Replacement fibrosis increases diastolic dysfunction and ventricular stiffness [17][10], and plays a role in the progression of heart failure.