Heavy metal (HM) toxicity has become a global concern in recent years and is imposing a severe threat to the environment and human health. In the case of plants, a higher concentration of HMs, above a threshold, adversely affects cellular metabolism because of the generation of reactive oxygen species (ROS) which target the key biological molecules. Moreover, some of the HMs such as mercury and arsenic, among others, can directly alter the protein/enzyme activities by targeting their –SH group to further impede the cellular metabolism. Particularly, inhibition of photosynthesis has been reported under HM toxicity because HMs trigger the degradation of chlorophyll molecules by enhancing the chlorophyllase activity and by replacing the central Mg ion in the porphyrin ring which affects overall plant growth and yield. Consequently, plants utilize various strategies to mitigate the negative impact of HM toxicity by limiting the uptake of these HMs and their sequestration into the vacuoles with the help of various molecules including proteins such as phytochelatins, metallothionein, compatible solutes, and secondary metabolites.
- heavy metals
- ROS
- redox status
- photosynthesis
- antioxidants
- sequestration
1. Introduction
2. Tolerance to HM Toxicity Is Mediated by a Complex Signaling Network
Plants under HM toxicity exhibit biochemical, physiological, and molecular changes as a part of their tolerance response, and understanding these responses is crucial to induce HM tolerance in sensitive crop plants (Figure 1).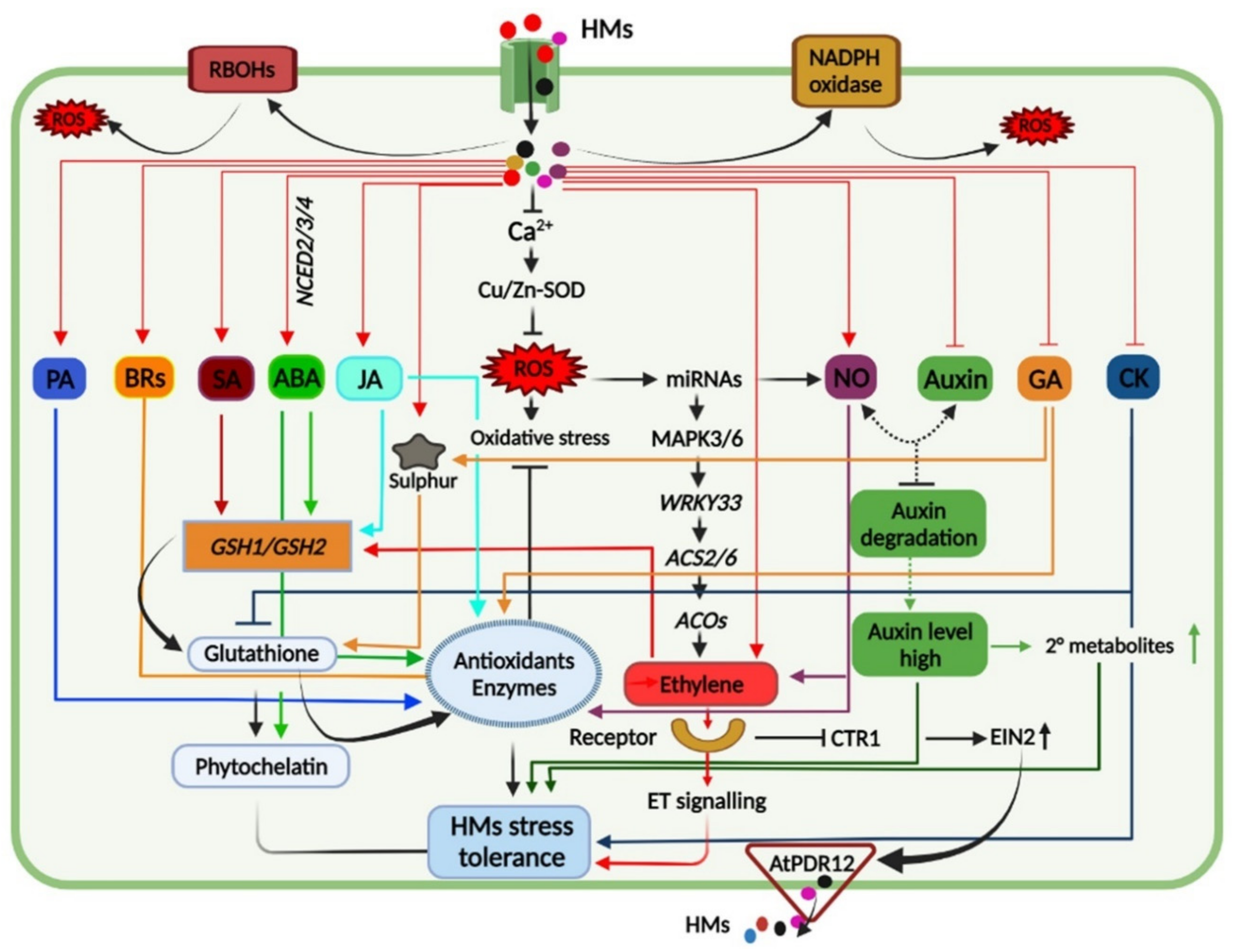
| Plant | Gene(s) | Metal(s) | Reported Phenotypes | References | ||||
|---|---|---|---|---|---|---|---|---|
| Lemna turonifera | AtNHX1 | Cadmium | vacuolar sequestration of metabolites and improved tolerance | Yao et al., 2020 | ||||
| Triticum aestivum L. | TaCATs | Arsenic | Stress tolerance | Tyagi et al., 2020 | ||||
| Oryza sativa | CCoAOMT | Copper | Lignin production and enhanced tolerance | Su et al., 2020 | ||||
| Oryza sativa | cadA and bmtA | Cadmium | Cd accumulation and Cd-nanoparticles (CdNPs) biosynthesis and improved tolerance by decreasing oxidative stress | Shi et al., 2020 | ||||
| Hordeum vulagare | HvPAL, HvMDH andHvCSY | Copper and Cobalt | Accumulation of phenolics and amino acids and increased tolerance | Lwalaba et al., 2020 | ||||
| Jatropha curcas | JcMT2a andJcPAL | Lead | Accumulation of antioxidants, e.g., flavonoids and phenolics and metal detoxification | Mohamed et al., 2020 | ||||
| Tobacco | EhMT1 | Copper | Decreased hydrogen peroxide (H | 2 | O | 2 | ) formation and increased tolerance | Xia et al., 2012 |
| Tobacco | TaMT3 | Cadmium | Increased superoxide dismutase (SOD) activity and conferred tolerance | Zhou et al., 2014 | ||||
| Tobacco | OSMT1e-p | Copper and Zinc | ROS scavenging and enhanced tolerance | Kumar et al., 2012 | ||||
| Arabidopsis thaliana | BjMT2 | Copper and Cadmium | Inhibits root elongation but increased tolerance | Zhigang et al., 2006 | ||||
| Hibiscus cannabinus L. | WRKY, GRAS, MYB, bHLH, ZFP, ERF, and NAC | Cadmium | Enhanced tolerance via molecular mechanism | Chen et al., 2020 | ||||
| Tobacco | NtCBP4 | Lead | Increased tolerance | Sunkar et al., 2000 | ||||
| Arabidopsis thaliana | ACBP1 | Lead | Higher gene expression and enhanced tolerance | Xiao et al., 2008; Du et al., 2015 | ||||
| Linum usitatissimum L. | LuACBP1 and LuACBP2 | Lead | Transcript level was higher in transgenic and improved tolerance | Pan et al., 2020 | ||||
| Oryza sativa | OsSTAR1 and OsSTAR2 | Aluminium | Decreased aluminium level in cell wall and enhanced tolerance | Huang et al., 2020 | ||||
| Fragaria vesca | FvABCC11 | Cadmium | Increased tolerance via ATP binding cassette (ABC) transporters | Shi et al., 2020 | ||||
| Arabidopsis thaliana | AtABCC3 and AtABCC6 | Cadmium | Phytochelatin mediated tolerance during seedling development | Brunetti et al., 2015; Gaillard et al., 2008 | ||||
| Oryza sativa | OsABCC1 | Arsenic | Increased tolerance via vacuolar sequestration | Song et al., 2014 | ||||
| Arabidopsis thaliana | AtABCC1 and AtABCC2 | Cadmium and Mercury | Enhanced tolerance via vacuolar sequestration | Park et al., 2012 | ||||
| Brassica napus | BnaABCC3 and BnaABCC4 | Cadmium | Enhanced stress tolerance | Zhang et al., 2018 | ||||
| Triticum aestivum | TaABCC | Cadmium | Distinct molecular expression and increased tolerance | Bhati et al., 2015 |
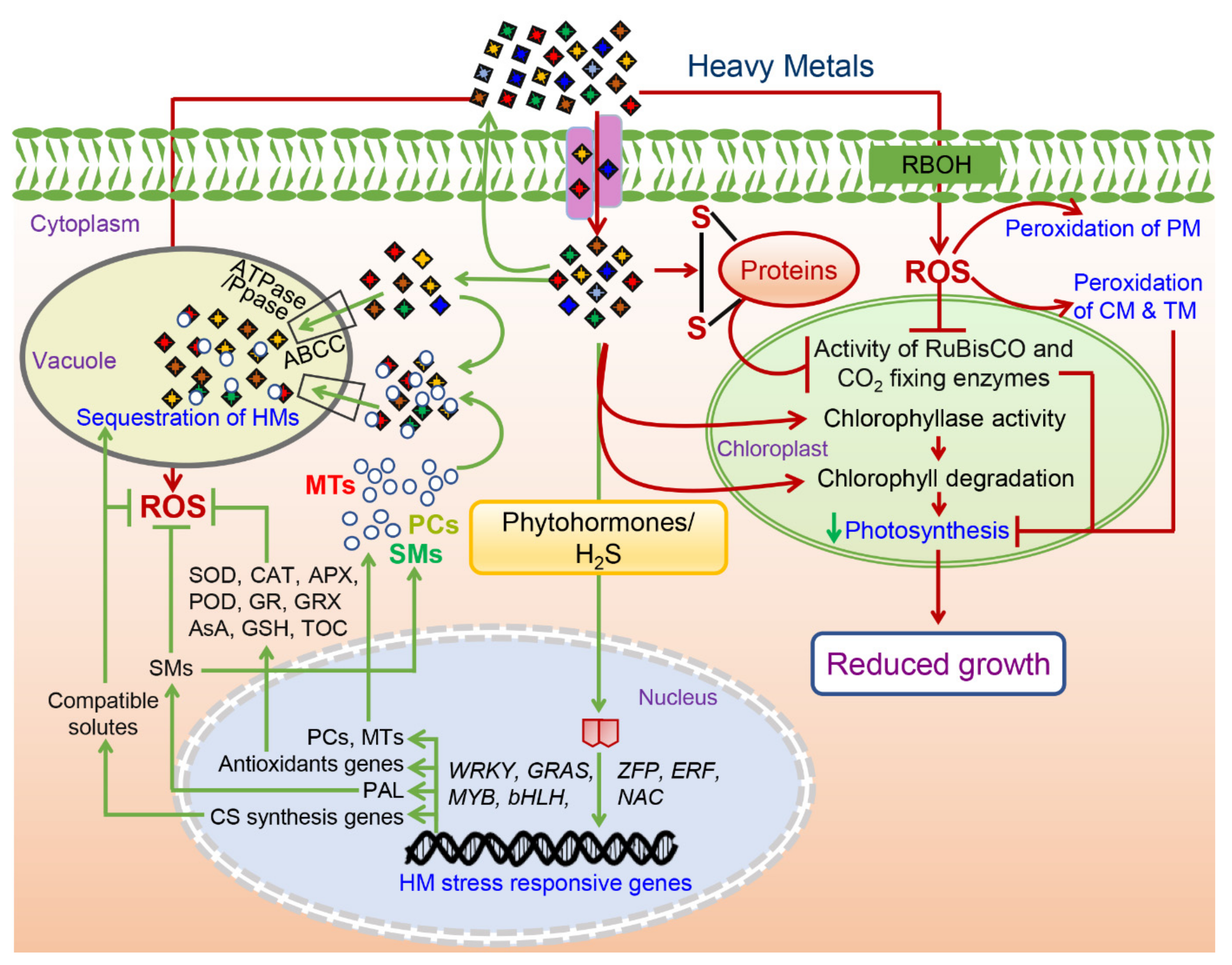 Figure 2. A putative diagram showing positive and positive molecular responses of the heavy metals (HM) toxicity in the plants. Responses marked with the red color represent negative effects of the HM toxicity while those marked with the green color represent tolerance response to alleviate the HM toxicity. Abbreviations: ROS—reactive oxygen species; SMs—secondary metabolites; CS—compatible solutes; PCs-phytochelatins; MTs—metallothioneins; SOD—superoxide dismutase; CAT—catalase; APX-ascorbate peroxidase; POD—peroxidase; GR—glutathione reductase; GRX—glutaredoxins; AsA—ascorbic acid; GSH—reduced glutathione; TOC—tocopherol; PAL—phenylalanine ammonia lyase; RBOH—respiratory burst oxidase homolog; PM—plasma membrane; CM—chloroplast membrane; TM—thylakoid membrane.
Figure 2. A putative diagram showing positive and positive molecular responses of the heavy metals (HM) toxicity in the plants. Responses marked with the red color represent negative effects of the HM toxicity while those marked with the green color represent tolerance response to alleviate the HM toxicity. Abbreviations: ROS—reactive oxygen species; SMs—secondary metabolites; CS—compatible solutes; PCs-phytochelatins; MTs—metallothioneins; SOD—superoxide dismutase; CAT—catalase; APX-ascorbate peroxidase; POD—peroxidase; GR—glutathione reductase; GRX—glutaredoxins; AsA—ascorbic acid; GSH—reduced glutathione; TOC—tocopherol; PAL—phenylalanine ammonia lyase; RBOH—respiratory burst oxidase homolog; PM—plasma membrane; CM—chloroplast membrane; TM—thylakoid membrane.
3. Sequestration and Compartmentalization: Plants’ Way to Alleviate the HM Toxicity
34. Conclusions
References
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2021, 1–15.
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216.
- Boyd, R.S.; Rajakaruna, N. Heavy Metal Tolerance; Oxford University Press: Oxford, UK, 2013.
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452.
- Yu, X.; Li, Y.; Zhang, C.; Liu, H.; Liu, J.; Zheng, W.; Kang, X.; Leng, X.; Zhao, K.; Gu, Y. Culturable heavy metal-resistant and plant growth promoting bacteria in V-Ti magnetite mine tailing soil from Panzhihua, China. PLoS ONE 2014, 9, e106618.
- Gill, M. Heavy metal stress in plants: A review. Int. J. Adv. Res. 2014, 2, 1043–1055.
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828.
- Seth, C.S.; Chaturvedi, P.K.; Misra, V. Toxic effect of arsenate and cadmium alone and in combination on giant duckweed (Spirodela polyrrhiza L.) in response to its accumulation. Environ. Toxicol. An. Int. J. 2007, 22, 539–549.
- Seth, C.S.; Misra, V.; Chauhan, L.K.S. Accumulation, detoxification, and genotoxicity of heavy metals in indian mustard (Brassica juncea L.). Int. J. Phytoremediation 2012, 14, 1–13.
- Ozturk, M.; Yucel, E.; Gucel, S.; Sakçali, S.; Aksoy, A. 28 Plants as Biomonitors of Trace Elements Pollution in Soil; Academia: San Francisco, CA, USA, 2008.
- Öztürk, M.; Ashraf, M.; Aksoy, A.; Ahmad, M.S.A.; Hakeem, K.R. Plants, Pollutants and Remediation; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9401771944.
- Hasan, M.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of plant proteins to heavy metal stress—a review. Front. Plant Sci. 2017, 8, 1492.
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702.
- López-Martín, M.C.; Becana, M.; Romero, L.C.; Gotor, C. Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in Arabidopsis. Plant Physiol. 2008, 147, 562–572.
- Xiao, S.; Gao, W.; Chen, Q.; Ramalingam, S.; Chye, M. Overexpression of membrane-associated acyl-CoA-binding protein ACBP1 enhances lead tolerance in Arabidopsis. Plant J. 2008, 54, 141–151.
- DU, Z.; CHEN, M.; CHEN, Q.; GU, J.; CHYE, M. Expression of A rabidopsis acyl-CoA-binding proteins AtACBP 1 and AtACBP 4 confers P b (II) accumulation in B rassica juncea roots. Plant Cell Environ. 2015, 38, 101–117.
- Sunkar, R.; Kaplan, B.; Bouché, N.; Arazi, T.; Dolev, D.; Talke, I.N.; Maathuis, F.J.M.; Sanders, D.; Bouchez, D.; Fromm, H. Expression of a truncated tobacco NtCBP4 channel in transgenic plants and disruption of the homologous Arabidopsis CNGC1 gene confer Pb2+ tolerance. Plant J. 2000, 24, 533–542.
- Ogawa, I.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Time course analysis of gene regulation under cadmium stress in rice. Plant Soil 2009, 325, 97–108.
- He, F.; Liu, Q.; Zheng, L.; Cui, Y.; Shen, Z.; Zheng, L. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front. Plant Sci. 2015, 6, 1136.
- Liu, Z.; Fang, H.; Pei, Y.; Jin, Z.; Zhang, L.; Liu, D. WRKY transcription factors down-regulate the expression of H 2 S-generating genes, LCD and DES in Arabidopsis thaliana. Sci. Bull. 2015, 60, 995–1001.
- Rui, H.; Zhang, X.; Shinwari, K.I.; Zheng, L.; Shen, Z. Comparative transcriptomic analysis of two Vicia sativa L. varieties with contrasting responses to cadmium stress reveals the important role of metal transporters in cadmium tolerance. Plant Soil 2018, 423, 241–255.
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome analysis revealed key genes and pathways related to cadmium-stress tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970.
- Weber, M.; Trampczynska, A.; Clemens, S. Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+−hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 2006, 29, 950–963.
- Liu, T.; Liu, S.; Guan, H.; Ma, L.; Chen, Z.; Gu, H.; Qu, L.-J. Transcriptional profiling of Arabidopsis seedlings in response to heavy metal lead (Pb). Environ. Exp. Bot. 2009, 67, 377–386.
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y. Zinc-finger transcription factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719.
- He, G.; Qin, L.; Tian, W.; Meng, L.; He, T.; Zhao, D. Heavy metal Transporters-Associated proteins in Solanum tuberosum: Genome-wide identification, comprehensive gene feature, evolution and expression analysis. Genes 2020, 11, 1269.
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719.
- Gill, S.S.; Anjum, N.A.; Ahmad, I.; Thangavel, P.; Sridevi, G.; Pacheco, M.; Duarte, A.C.; Umar, S.; Khan, N.A.; Pereira, M.E. Metal hyperaccumulation and tolerance in Alyssum, Arabidopsis and Thlaspi: An overview. Plant Fam. Brassicaceae 2012, 21, 99–137.
- Arslan Topal, E.I.; Topal, M.; Öbek, E. Assessment of heavy metal accumulations and health risk potentials in tomatoes grown in the discharge area of a municipal wastewater treatment plant. Int. J. Environ. Health Res. 2020, 1–13.
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486.
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182.
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192.
- Frey, B.; Keller, C.; Zierold, K. Distribution of Zn in functionally different leaf epidermal cells of the hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2000, 23, 675–687.
- Zhao, F.J.; Lombi, E.; Breedon, T. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 2000, 23, 507–514.
- Küpper, H.; Lombi, E.; Zhao, F.-J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84.
- Hu, P.-J.; Qiu, R.-L.; Senthilkumar, P.; Jiang, D.; Chen, Z.-W.; Tang, Y.-T.; Liu, F.-J. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ. Exp. Bot. 2009, 66, 317–325.
- Brooks, R.R.; Reeves, R.D.; Morrison, R.S.; Malaisse, F. Hyperaccumulation of copper and cobalt—A review. Bull. la Société R. Bot. Belgique/Bulletin van K. Belgische Bot. Ver. 1980, 113, 166–172.
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433.
- Chardonnens, A.N.; Ten Bookum, W.M.; Kuijper, L.D.J.; Verkleij, J.A.C.; Ernst, W.H.O. Distribution of cadmium in leaves of cadmium tolerant and sensitive ecotypes of Silene vulgaris. Physiol. Plant. 1998, 104, 75–80.
- Masood, A.; Iqbal, N.; Khan, N.A. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012, 35, 524–533.
- Carrier, P.; Baryla, A.; Havaux, M. Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium-contaminated soil. Planta 2003, 216, 939–950.
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359.
- Sobrino-Plata, J.; Ortega-Villasante, C.; Flores-Cáceres, M.L.; Escobar, C.; Del Campo, F.F.; Hernández, L.E. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 2009, 77, 946–954.
- Thangavel, P.; Long, S.; Minocha, R. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell. Tissue Organ Cult. 2007, 88, 201–216.
- Dago, À.; González, I.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Chemometrics applied to the analysis of induced phytochelatins in Hordeum vulgare plants stressed with various toxic non-essential metals and metalloids. Talanta 2014, 118, 201–209.
- Wang, F.; Wang, Z.; Zhu, C. Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim. Biophys. Sin. 2012, 44, 886–893.
- Zhigang, A.; Cuijie, L.; Yuangang, Z.; Yejie, D.; Wachter, A.; Gromes, R.; Rausch, T. Expression of BjMT2, a metallothionein 2 from Brassica juncea, increases copper and cadmium tolerance in Escherichia coli and Arabidopsis thaliana, but inhibits root elongation in Arabidopsis thaliana seedlings. J. Exp. Bot. 2006, 57, 3575–3582.
- Kumar, G.; Kushwaha, H.R.; Panjabi-Sabharwal, V.; Kumari, S.; Joshi, R.; Karan, R.; Mittal, S.; Pareek, S.L.S.; Pareek, A. Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol. 2012, 12, 107.
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120.
- Zhou, B.; Yao, W.; Wang, S.; Wang, X.; Jiang, T. The metallothionein gene, TaMT3, from Tamarix androssowii confers Cd2+ tolerance in tobacco. Int. J. Mol. Sci. 2014, 15, 10398–10409.
- Xia, Y.; Qi, Y.; Yuan, Y.; Wang, G.; Cui, J.; Chen, Y.; Zhang, H.; Shen, Z. Overexpression of Elsholtzia haichowensis metallothionein 1 (EhMT1) in tobacco plants enhances copper tolerance and accumulation in root cytoplasm and decreases hydrogen peroxide production. J. Hazard. Mater. 2012, 233, 65–71.
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon mechanisms to ameliorate heavy metal stress in plants. Biomed. Res. Int. 2018, 2018, 8492898.
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1443.
- Shi, G.; Cai, Q.; Liu, C.; Wu, L. Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul. 2010, 61, 45–52.
- Song, A.; Li, Z.; Zhang, J.; Xue, G.; Fan, F.; Liang, Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J. Hazard. Mater. 2009, 172, 74–83.
- Feng, J.; Shi, Q.; Wang, X.; Wei, M.; Yang, F.; Xu, H. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. 2010, 123, 521–530.
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249.
- Su, N.; Ling, F.; Xing, A.; Zhao, H.; Zhu, Y.; Wang, Y.; Deng, X.; Wang, C.; Xu, X.; Hu, Z. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020, 175, 104059.
- Kupper, H.; Mijovilovich, A.; Meyer-Klaucke, W.; Kroneck, P.M.H. Tissue-and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757.
- Mohamed, A.A.A.; Dardiry, M.H.O.; Samad, A.; Abdelrady, E. Exposure to Lead (Pb) Induced Changes in the Metabolite Content, Antioxidant Activity and Growth of Jatropha curcas (L.). Trop. Plant Biol. 2020, 13, 150–161.
- Xu, J.; Zhu, Y.; Ge, Q.; Li, Y.; Sun, J.; Zhang, Y.; Liu, X. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012, 196, 125–138.
- Lwalaba, J.L.W.; Zvobgo, G.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Kirika, B.A.; Tshibangu, A.K.; Adil, M.F.; Sehar, S.; Mukobo, R.P. High accumulation of phenolics and amino acids confers tolerance to the combined stress of cobalt and copper in barley (Hordeum vulagare). Plant Physiol. Biochem. 2020, 155, 927–937.
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776.
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204.
- Song, W.-Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.-H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Ma, J.F. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704.
- Rea, P.A. MRP subfamily ABC transporters from plants and yeast. J. Exp. Bot. 1999, 50, 895–913.
- Martinoia, E.; Klein, M.; Geisler, M.; Bovet, L.; Forestier, C.; Kolukisaoglu, U.; MuÈller-RoÈber, B.; Schulz, B. Multifunctionality of plant ABC transporters–more than just detoxifiers. Planta 2002, 214, 345–355.
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2020, 302, 110733.
- Bhati, K.K.; Sharma, S.; Aggarwal, S.; Kaur, M.; Shukla, V.; Kaur, J.; Mantri, S.; Pandey, A.K. Genome-wide identification and expression characterization of ABCC-MRP transporters in hexaploid wheat. Front. Plant Sci. 2015, 6, 488.
- Zhang, X.D.; Zhao, K.X.; Yang, Z.M. Identification of genomic ATP binding cassette (ABC) transporter genes and Cd-responsive ABCs in Brassica napus. Gene 2018, 664, 139–151.
- Gaillard, S.; Jacquet, H.; Vavasseur, A.; Leonhardt, N.; Forestier, C. AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and up-regulated by cadmium in Arabidopsis thaliana. BMC Plant Biol. 2008, 8, 22.
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829.
- Shi, M.; Wang, S.; Zhang, Y.; Wang, S.; Zhao, J.; Feng, H.; Sun, P.; Fang, C.; Xie, X. Genome-wide characterization and expression analysis of ATP-binding cassette (ABC) transporters in strawberry reveal the role of FvABCC11 in cadmium tolerance. Sci. Hortic. 2020, 271, 109464.
- Park, J.; Song, W.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288.
- Kim, D.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218.
- Qiao, C.; Yang, J.; Wan, Y.; Xiang, S.; Guan, M.; Du, H.; Tang, Z.; Lu, K.; Li, J.; Qu, C. A Genome-Wide Survey of MATE Transporters in Brassicaceae and Unveiling Their Expression Profiles under Abiotic Stress in Rapeseed. Plants 2020, 9, 1072.
- Yao, J.; Sun, J.; Chen, Y.; Shi, L.; Yang, L.; Wang, Y. The molecular mechanism underlying cadmium resistance in NHX1 transgenic Lemna turonifera was studied by comparative transcriptome analysis. Plant Cell Tissue Organ Cult. 2020, 143, 189–200.
- Zhang, X.D.; Meng, J.G.; Zhao, K.X.; Chen, X.; Yang, Z.M. Annotation and characterization of Cd-responsive metal transporter genes in rapeseed (Brassica napus). Biometals 2018, 31, 107–121.
- Komal, T.; Mustafa, M.; Ali, Z.; Kazi, A.G. Heavy metal uptake and transport in plants. In Heavy Metal Contamination of Soils; Springer: Berlin/Heidelberg, Germany, 2015; pp. 181–194.
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233.
- Connolly, E.L.; Fett, J.P.; Guerinot, M. Lou Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357.
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381.
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition metal transport in plants and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088.
- Van der Zaal, B.J.; Neuteboom, L.W.; Pinas, J.E.; Chardonnens, A.N.; Schat, H.; Verkleij, J.A.C.; Hooykaas, P.J.J. Overexpression of a novel Arabidopsis gene related to putative zinc-transporter genes from animals can lead to enhanced zinc resistance and accumulation. Plant Physiol. 1999, 119, 1047–1056.
- Assunção, A.G.L.; Martins, P.D.C.; De Folter, S.; Vooijs, R.; Schat, H.; Aarts, M.G.M. Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2001, 24, 217–226.
- Delhaize, E.; Kataoka, T.; Hebb, D.M.; White, R.G.; Ryan, P.R. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell 2003, 15, 1131–1142.
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312.
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of antioxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol. Environ. Saf. 2018, 147, 382–393.
- Bali, S.; Jamwal, V.L.; Kaur, P.; Kohli, S.K.; Ohri, P.; Gandhi, S.G.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ahmad, P. Role of P-type ATPase metal transporters and plant immunity induced by jasmonic acid against Lead (Pb) toxicity in tomato. Ecotoxicol. Environ. Saf. 2019, 174, 283–294.
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12.
- Chaturvedi, R.; Varun, M.; Paul, M.S. Phytoremediation: Uptake and role of metal transporters in some members of Brassicaceae. In Phytoremediation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 453–468.
- Ricachenevsky, F.K.; Menguer, P.K.; Sperotto, R.A.; Williams, L.E.; Fett, J.P. Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Front. Plant Sci. 2013, 4, 144.
